Label: HEMORRHOIDAL CREAM- glycerol, phenylephine, pramoxine, petrolatum cream
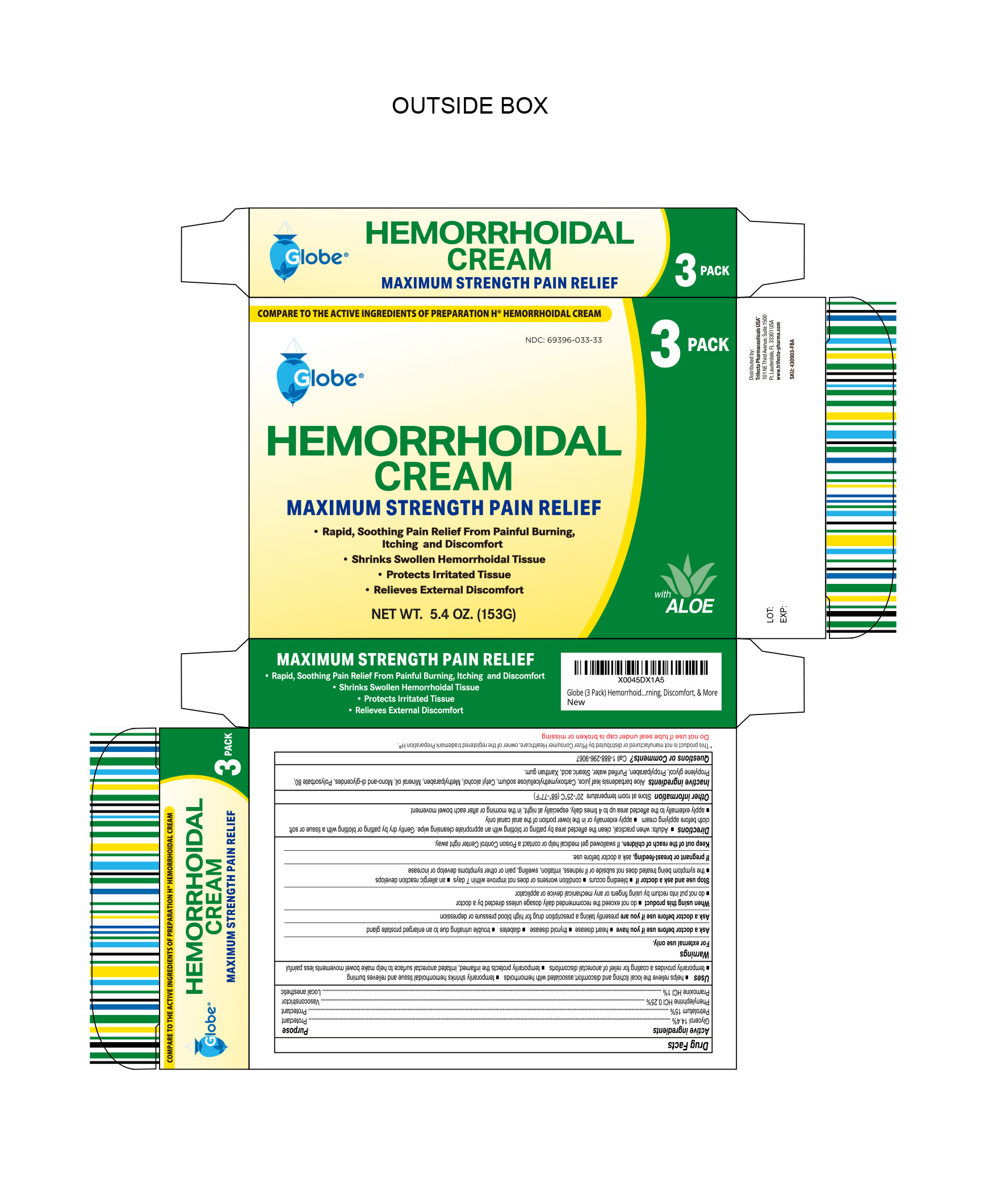
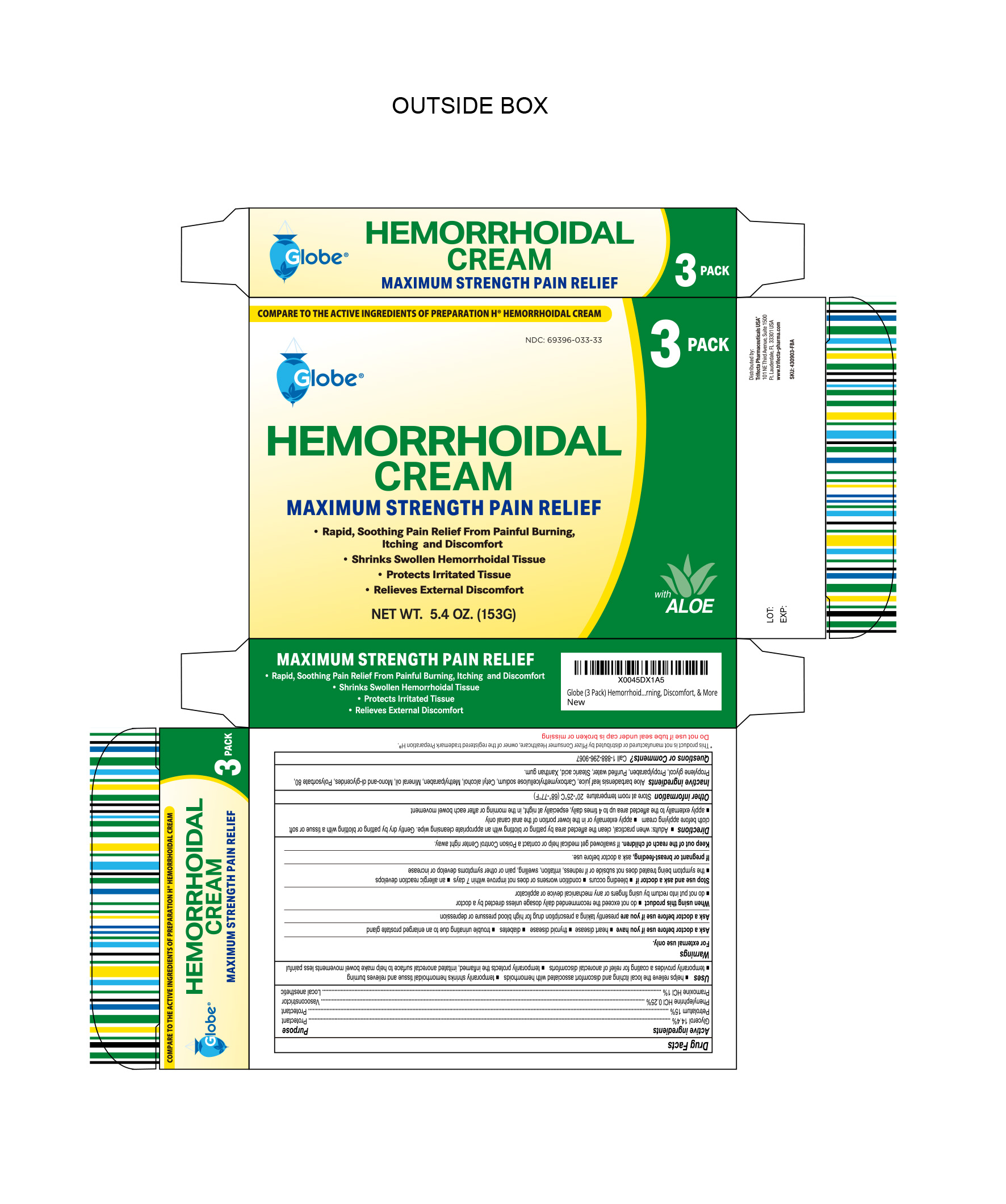
- NDC Code(s): 69396-033-18, 69396-033-33
- Packager: Trifecta Pharmaceuticals Usa Llc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
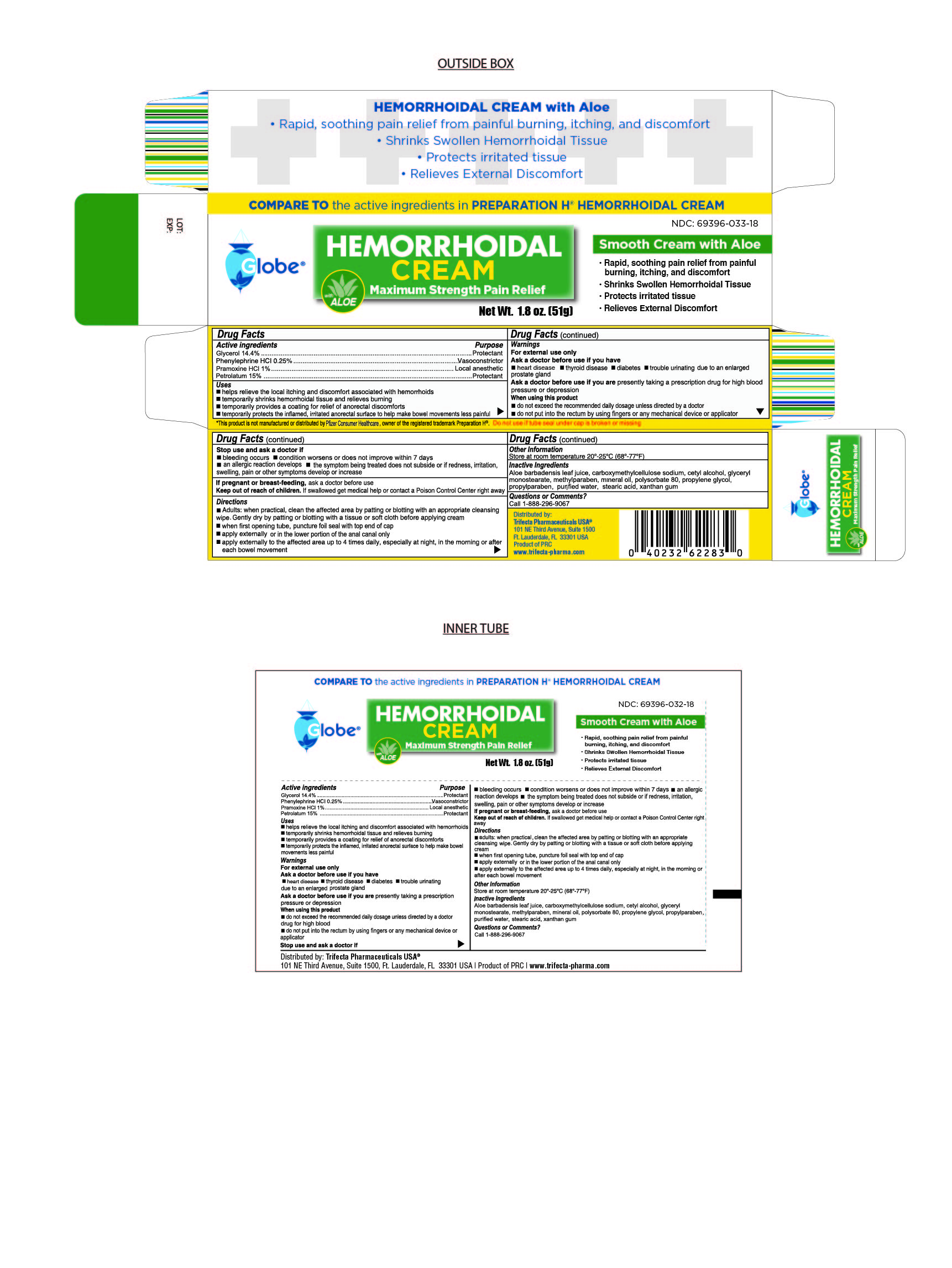
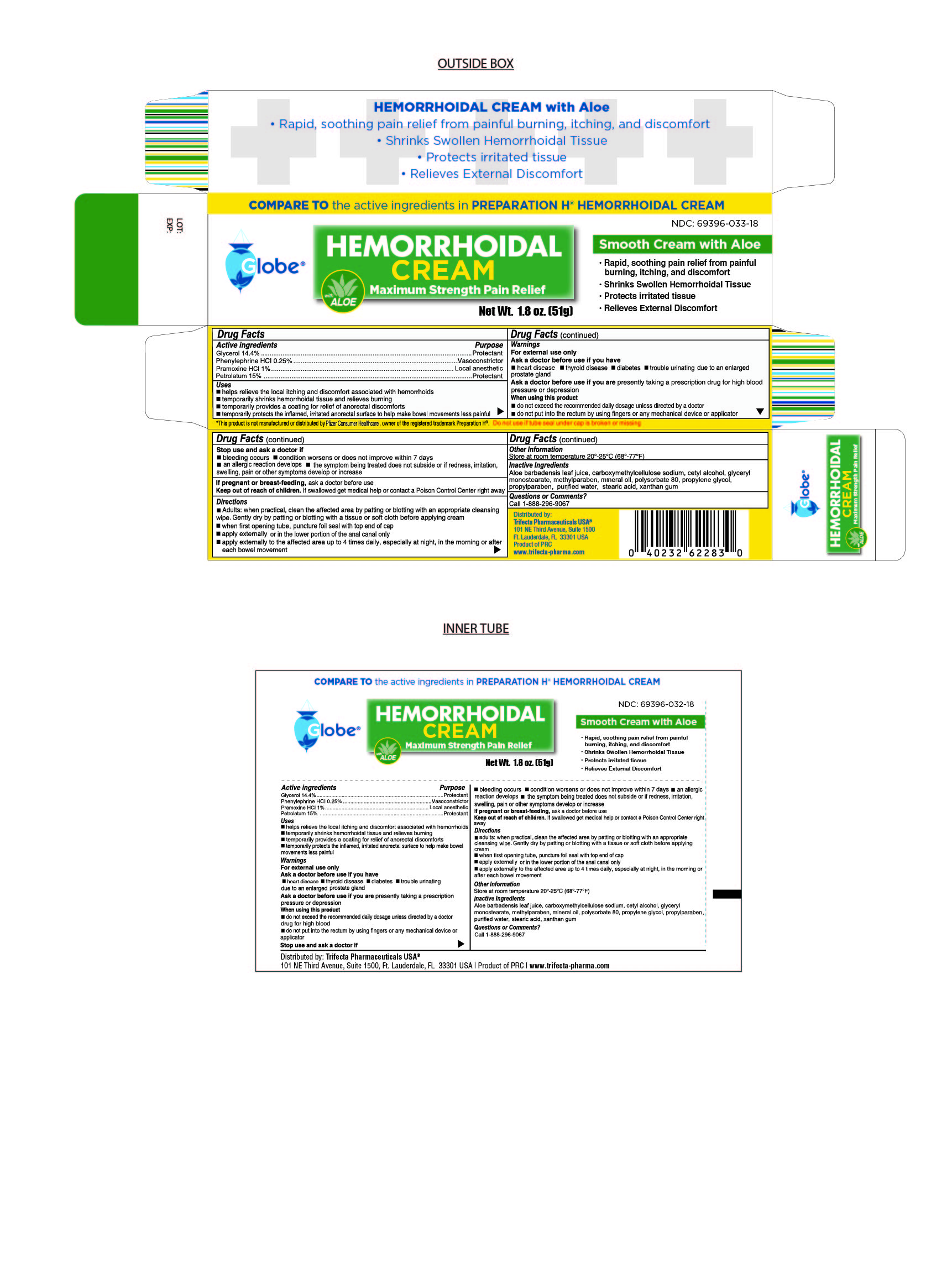
- Drug Facts
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
-
Uses
Helps relieve the local itching and discomfort associated with hemorrhoids
Temporarily shrinks hemorrhoidal tissue and relieves burning
Temporarily provides a coating for relief of anorectal discomforts
Temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful
- Stop use and ask a doctor
- If Pregnant or Breast Feeding
- Keep out of reach of children
-
Directions
Adults:
When practical, clean the affected area by patting or blotting with an appropriate cleaning wipe. Gently dry by patting or blotting with a tissue or soft cloth before applying cream.
When first opening tube, puncture foil seal with top end of cap
Apply externally or in the lower portion of the anal canal only
Apply externally to the affected area up to 4 times daily, especially at night, in the morning or after each bowel movement
-
Warnings
For External Use Only
Ask doctor brfore use if you have
Heart Disease
Thyroid Disease
Diabetes
Trouble urinating due to an enlarged prostate gland
Ask doctor before use if you are presently taking a prescription for high blood pressure or depression
When using this product
Do not exceed the recommended daily dosage unless directed by a doctor
Do not put into the rectum by using fingers or any mechanical device or applicator
- Other Information
- Inactive Ingredients
- Questions or Comments
- Distributed By
- Packaging
- Label
-
INGREDIENTS AND APPEARANCE
HEMORRHOIDAL CREAM
glycerol, phenylephine, pramoxine, petrolatum creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69396-033 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 1 g in 100 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 15 g in 100 g GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 14.4 g in 100 g PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 0.25 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CARBOXYMETHYLCELLULOSE SODIUM (0.7 CARBOXYMETHYL SUBSTITUTION PER SACCHARIDE; 100-200 MPA.S AT 1%) (UNII: 99H65D77XY) CETYL ALCOHOL (UNII: 936JST6JCN) PROPYLPARABEN (UNII: Z8IX2SC1OH) MINERAL OIL (UNII: T5L8T28FGP) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) STEARIC ACID (UNII: 4ELV7Z65AP) XANTHAN GUM (UNII: TTV12P4NEE) METHYLPARABEN (UNII: A2I8C7HI9T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69396-033-18 1 in 1 BOX 01/25/2019 1 51 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:69396-033-33 3 in 1 BOX 06/14/2024 2 51 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 01/25/2019 Labeler - Trifecta Pharmaceuticals Usa Llc (079424163)