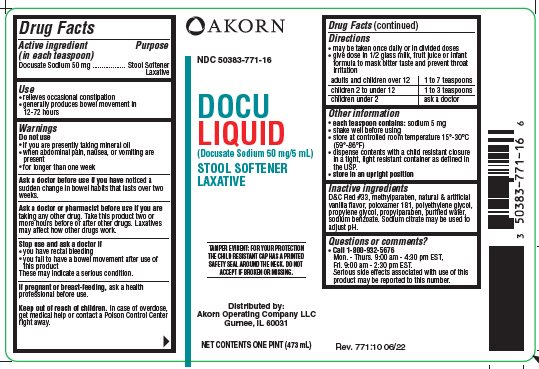
Label: DOCU LIQUID- docusate sodium liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 50383-349-10, 50383-349-11, 50383-771-16 - Packager: Akorn Operating Company LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 13, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Uses
-
Warnings
Do Not Use
- •
- if you are presently taking mineral oil
- •
- when abdominal pain, nausea, or vomiting are present
- •
- for longer than one week
Ask a doctor before use if you have
noticed a sudden change in bowel habits that lasts over two weeks.
Ask a doctor or pharmacist before use if you are
taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
- Directions
- Other information
- Inactive Ingredients
- Questions or comments?
-
Package/Label Principal Display Panel
NDC 50383-771-16
DOCU LIQUID
(Docusate Sodium 50 mg/5 mL)
STOOL SOFTENER LAXATIVE
TAMPER EVIDENT: FOR YOUR PROTECTION THE CHILD RESISTANT CAP HAS A PRINTED SAFETY SEAL AROUND THE NECK. DO NOT ACCEPT IF BROKEN OR MISSING.
Distributed by:
Akorn Operating Company LLC
Gurnee, IL 60031
NET CONTENTS ONE PINT (473 mL)
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
DOCU LIQUID
docusate sodium liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50383-771 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg in 5 mL Inactive Ingredients Ingredient Name Strength D&C RED NO. 33 (UNII: 9DBA0SBB0L) METHYLPARABEN (UNII: A2I8C7HI9T) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) WATER (UNII: 059QF0KO0R) POLOXAMER 181 (UNII: 09Y8E6164A) Product Characteristics Color PINK Score Shape Size Flavor VANILLA (natural and artificial flavor) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50383-771-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/01/1997 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 08/01/1997 DOCU LIQUID
docusate sodium liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50383-349 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 50 mg in 5 mL Inactive Ingredients Ingredient Name Strength D&C RED NO. 33 (UNII: 9DBA0SBB0L) METHYLPARABEN (UNII: A2I8C7HI9T) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) WATER (UNII: 059QF0KO0R) POLOXAMER 181 (UNII: 09Y8E6164A) Product Characteristics Color PINK Score Shape Size Flavor VANILLA (natural and artificial flavor) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50383-349-11 10 in 1 CASE 05/28/2021 1 10 in 1 TRAY 1 NDC:50383-349-10 10 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part334 08/01/1997 Labeler - Akorn Operating Company LLC (117696873) Registrant - Akorn Operating Company LLC (117693100) Establishment Name Address ID/FEI Business Operations Akorn Operating Company LLC 117696873 MANUFACTURE(50383-771, 50383-349) , PACK(50383-771, 50383-349)