Label: FOLIVANE-PLUS WITH ASCORBIC ACID PRECURSORS- ascorbic acid, thiamine mononitrate, riboflavin, niacin, pyridoxine hcl, folic acid, cyanocobalamin, biotin, d-calcium pantothenate, ferrous fumarate, and polysaccharide iron complex capsule
- NHRIC Code(s): 13811-539-90
- Packager: Trigen Laboratories, LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated January 7, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
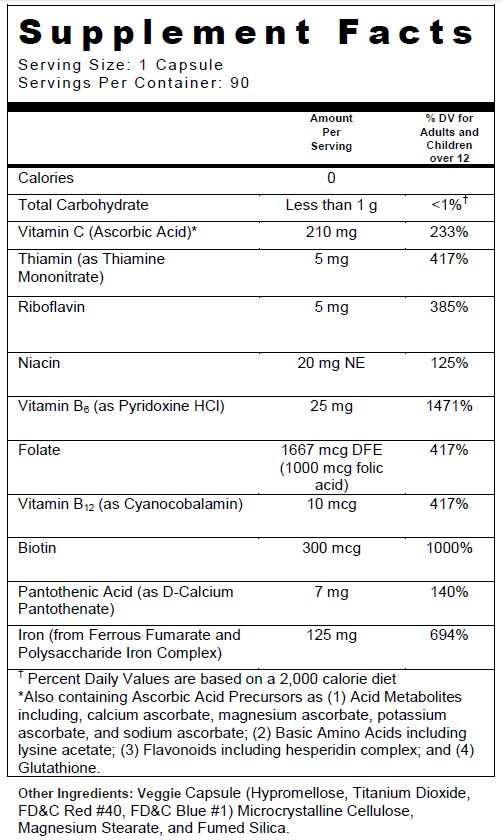
- SUPPLEMENT FACTS
-
CONTRAINDICATIONS
Folivane™ - Plus is contraindicated in patients with known hypersensitivity to any of its ingredients; also, all iron compounds are contraindicated in patients with hemosiderosis, hemochromatosis, or hemolytic anemias. Pernicious anemia is a contraindication, as folic acid may obscure its signs and symptoms.
- WARNINGS
-
PRECAUTIONS
General: Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where B12 is deficient. Folic acid in doses above 0.1 mg daily may obscure pernicious anemia assessment, such that hematologic remission can occur while neurological manifestations remain progressive.
Biotin levels higher than the recommended daily allowance may cause interference with some laboratory tests, including lead to incorrect test results. Tell your healthcare provider about all prescription and over-the-counter medicines, vitamins, and dietary supplements that you take, including biotin.Pediatric Use: Safety and effectiveness of this product have not been established in pediatric patients.
Geriatric Use: Safety and effectiveness of this product have not been established in elderly patients.
- DRUG INTERACTIONS
-
ADVERSE REACTIONS
Folic Acid: Allergic sensitizations have been reported following both oral and parenteral administration of folic acid.
Ferrous Fumarate: Gastrointestinal disturbances (anorexia, nausea, diarrhea, constipation) occur occasionally, but are usually mild and may subside with continuation of therapy. Although the absorption of iron is best when taken between meals, taking Folivane™ - Plus after meals may diminish occasional G.I. disturbances. Folivane™ - Plus is best absorbed when taken at bedtime.
-
OVERDOSAGE
Acute overdosage of iron may cause abdominal pain, nausea and vomiting and, in severe cases, cardiovascular collapse and death. Other more chronic symptoms include pallor and cyanosis, melena, shock, drowsiness and coma. The estimated overdose of orally ingested iron is 300 mg/kg body weight. Toxic effects are seen at 10-20 mg/kg elemental iron. When overdoses are ingested by children, severe reactions, including fatalities, have resulted. Folivane™-Plus should be stored beyond the reach of children to prevent against accidental iron poisoning. Keep this product out of the reach of children.
Treatment: For specific therapy, exchange transfusion and chelating agents should be used. For general management, perform gastric lavage with sodium bicarbonate solution or milk. Administer intravenous fluids and electrolytes and use oxygen.
- DESCRIPTION
- DIRECTIONS FOR USE
- HOW SUPPLIED
- STORAGE
-
HEALTH CLAIM
KEEP OUT OF REACH OF CHILDREN.
For use on the order of a healthcare practitioner.
Call your doctor about side effects. To report side effects, call Trigen Laboratories at 1-770-509-4500 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
PLR-FOLPLUS-00001-1 Rev. 12/2021
Manufactured For:
Trigen Laboratories, LLC
Alpharetta, GA 30005 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FOLIVANE-PLUS WITH ASCORBIC ACID PRECURSORS
ascorbic acid, thiamine mononitrate, riboflavin, niacin, pyridoxine hcl, folic acid, cyanocobalamin, biotin, d-calcium pantothenate, ferrous fumarate, and polysaccharide iron complex capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:13811-539 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 62.5 mg IRON (UNII: E1UOL152H7) (IRON - UNII:E1UOL152H7) IRON 62.5 mg ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 210 mg THIAMINE MONONITRATE (UNII: 8K0I04919X) (Thiamine ION - UNII:4ABT0J945J) THIAMINE 5 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 5 mg NIACIN (UNII: 2679MF687A) (NIACIN - UNII:2679MF687A) NIACIN 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 25 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 10 ug BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 300 ug CALCIUM PANTOTHENATE (UNII: 568ET80C3D) (PANTOTHENIC ACID - UNII:19F5HK2737) PANTOTHENIC ACID 7 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSES (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:13811-539-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 10/01/2017 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color imprint scoring 1 shape size (solid drugs) 23 mm Labeler - Trigen Laboratories, LLC (830479668)