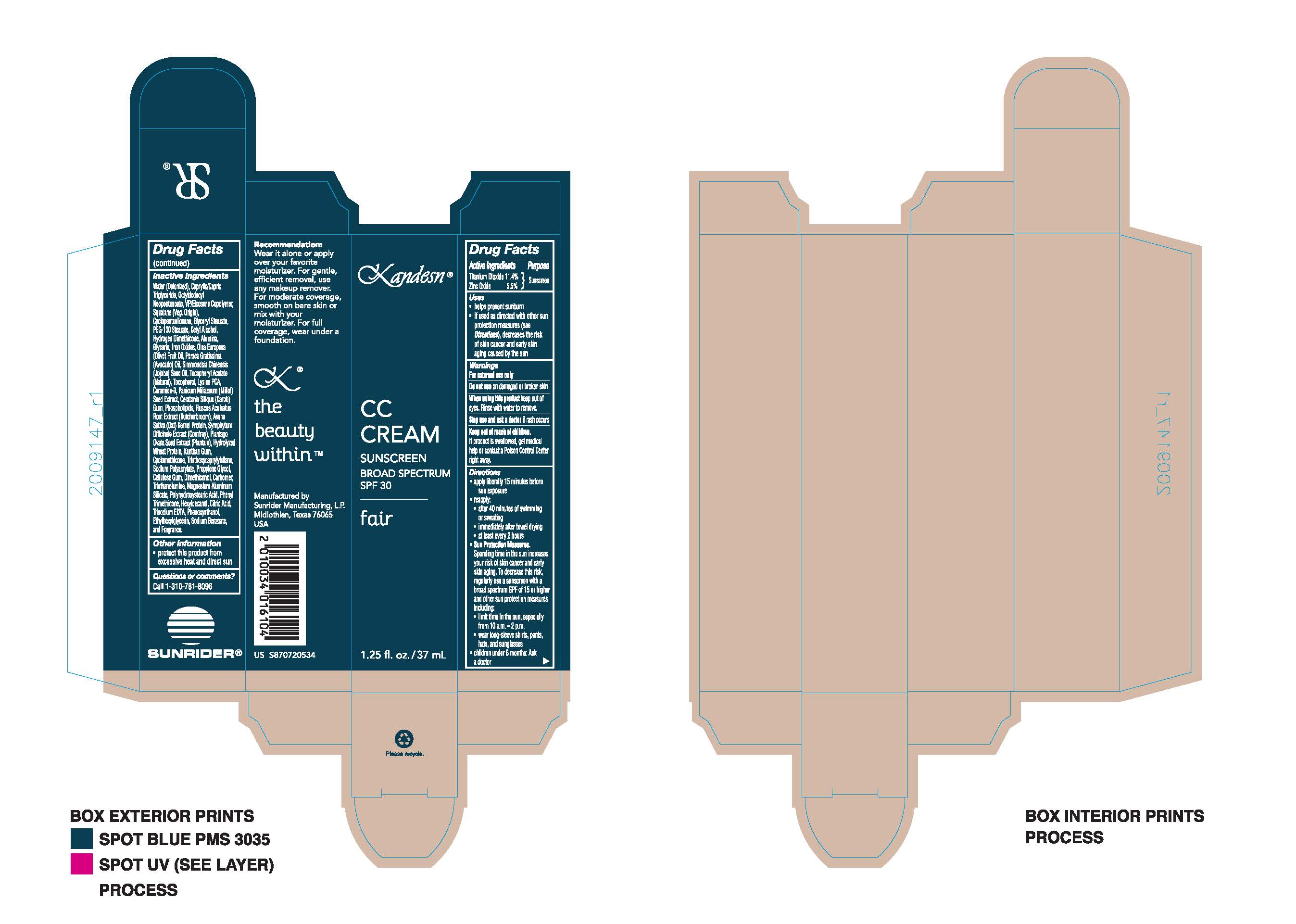
Label: KANDESN CC CREAM FAIR- sunscreen broad spectrum spf30 cream
- NDC Code(s): 62191-118-01
- Packager: Sunrider Manufacturing L.P.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
-
DOSAGE & ADMINISTRATION
DIRECTIONS:
- apply liberally 15 minutes before sun exposure
- Reapply:
- after 40 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, expecially from 10 a.m. - 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
OTHER INGREDIENTS:
Water (Deionized)
Caprylic/Capric
Triglyceride, Octyldodecyl
Neopentanoate, VP/Eicosene Copolymer,
Squalane (Veg. Origin),
Cyclopentasiloxane, Glyceryl Stearate,
PEG-100 Stearate, Cetyl Alcohol,
Hydrogen Dimethicone, Alumina,
Glycerin, Iron Oxides, Olea Europaea
(Olive) Fruit Oil, Persea Gratissima
(Avocado) Oil, Simmondsia Chinensis
(Jojoba) Seed Oil, Tocopheryl Acetate
(Natural), Tocopherol, Lysine PCA,
Ceramide-3, Panicum Miliaceum (Millet)
Seed Extract, Ceratonia Siliqua (Carob)
Gum, Phospholipids, Ruscus Aculeatus
Root Extract (Butcherbroom), Avena
Sativa (Oat) Kernel Protein, Symphytum
Officinale Extract (Comfrey), Plantago
Ovata Seed Extract (Plantain), Hydrolyzed
Wheat Protein, Xanthan Gum,
Cyclomethicone, Triethoxycaprylylsilane,
Sodium Polyacrylate, Propylene Glycol,
Cellulose Gum, Dimethiconol, Carbomer,
Triethanolamine, Magnesium Aluminum
Silicate, Polyhydroxystearic Acid, Phenyl
Trimethicone, Hexyldecanol, Citric Acid,
Trisodium EDTA, Phenoxyethanol,
Ethylhexylglycerin, Sodium Benzoate,
and Fragrance. -
WARNINGS
WARNINGS:
FOR EXTERNAL USE ONLY
Do not use on damaged or broken skin
When using this product keep oout of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs.
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
- KEEP OUT OF REACH OF CHILDREN
- Package and label
-
INGREDIENTS AND APPEARANCE
KANDESN CC CREAM FAIR
sunscreen broad spectrum spf30 creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62191-118 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 114 mg in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 55 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) SQUALANE (UNII: GW89575KF9) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) CETYL ALCOHOL (UNII: 936JST6JCN) METHICONE (20 CST) (UNII: 6777U11MKT) ALUMINUM OXIDE (UNII: LMI26O6933) OLIVE OIL (UNII: 6UYK2W1W1E) AVOCADO OIL (UNII: 6VNO72PFC1) JOJOBA OIL (UNII: 724GKU717M) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PHENOXYETHANOL (UNII: HIE492ZZ3T) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) HEXYLDECANOL (UNII: 151Z7P1317) CYCLOMETHICONE (UNII: NMQ347994Z) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE TRISODIUM (UNII: 420IP921MB) XANTHAN GUM (UNII: TTV12P4NEE) GLYCERIN (UNII: PDC6A3C0OX) RUSCUS ACULEATUS ROOT (UNII: ZW12V95I1Q) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) .DELTA.-TOCOPHEROL (UNII: JU84X1II0N) LYSINE PIDOLATE (UNII: 89WUG4A348) Ceramide NP (UNII: 4370DF050B) MILLET (UNII: TJR6B3R47P) SODIUM BENZOATE (UNII: OJ245FE5EU) DIMETHICONOL (40 CST) (UNII: 343C7U75XW) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) Oatmeal (UNII: 8PI54V663Y) SYMPHYTUM X UPLANDICUM LEAF (UNII: D05HXK6R3G) PLANTAGO OVATA SEED (UNII: UD50RBY30F) HYDROLYZED WHEAT PROTEIN (ENZYMATIC, 3000 MW) (UNII: J2S07SB0YL) LOCUST BEAN GUM (UNII: V4716MY704) FERRIC OXIDE RED (UNII: 1K09F3G675) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62191-118-01 1 in 1 PACKAGE 12/20/2021 1 41.75 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug part352 09/01/2016 Labeler - Sunrider Manufacturing L.P. (786951475)