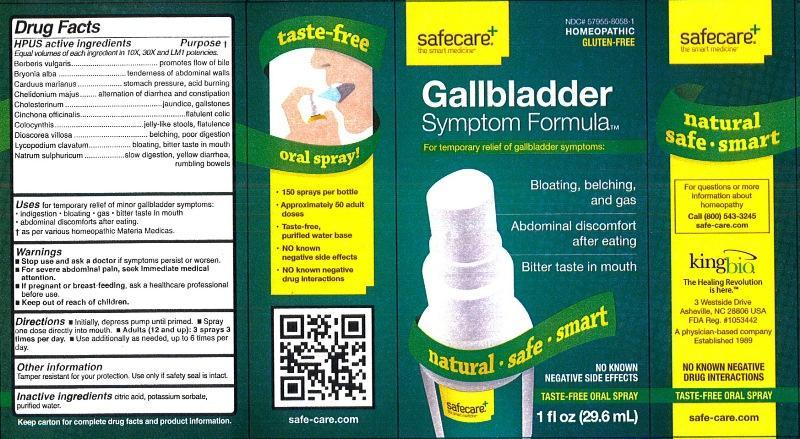
Label: GALLBLADDER SYMPTOM FORMULA- berberis vulgaris, bryonia alba, carduus marianus, chelidonium majus, cholesterinum, cinchona officinalis, colocynthis, dioscorea villosa, lycopodium clavatum and natrum sulphuricum liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 57955-8058-1 - Packager: King Bio Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated February 20, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Inactive Ingredients
-
Purpose
HPUS active ingredients
Equal volumes of each ingredient in 10X, 30X and LM1 potencies.
Berberis vulgaris..........................................promotes flow of bile
Bryonia alba.................................................tenderness of abdominal walls
Carduus marianus.......................................stomach pressure, acid burning
Chelidonium majus......................................alternation of diarrhea and constipation
Cholesterinum.............................................jaundice, gallstones
Cinchona officinalis......................................flatulent colic
Colocynthis..................................................jelly-like stools, flatulence
Dioscorea villosa..........................................belching, poor digestion
Lycopodium clavatum...................................bloating, bitter taste in mouth
Natrum sulphuricum.....................................slow digestion, yellow diarrhea, rumbling bowels
- Indications and Usage
-
Warnings
- Stop use and ask a doctor if symptoms persist or worsen.
- For severe abdominal pain, seek immediate medical attention.
- If pregnant or breast-feeding, ask a healthcare professional before use.
- Keep out of reach of children.
Other Information
Tamper resistant for your protection. Use only if safety seal is intact.
- KEEP OUT OF REACH OF CHILDREN
- Dosage and Administration
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GALLBLADDER SYMPTOM FORMULA
berberis vulgaris, bryonia alba, carduus marianus, chelidonium majus, cholesterinum, cinchona officinalis, colocynthis, dioscorea villosa, lycopodium clavatum and natrum sulphuricum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:57955-8058 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 10 [hp_X] in 29.6 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 10 [hp_X] in 29.6 mL SILYBUM MARIANUM SEED (UNII: U946SH95EE) (SILYBUM MARIANUM SEED - UNII:U946SH95EE) SILYBUM MARIANUM SEED 10 [hp_X] in 29.6 mL CHELIDONIUM MAJUS (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS 10 [hp_X] in 29.6 mL CHOLESTEROL (UNII: 97C5T2UQ7J) (CHOLESTEROL - UNII:97C5T2UQ7J) CHOLESTEROL 10 [hp_X] in 29.6 mL CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 10 [hp_X] in 29.6 mL CITRULLUS COLOCYNTHIS FRUIT PULP (UNII: 23H32AOH17) (CITRULLUS COLOCYNTHIS FRUIT PULP - UNII:23H32AOH17) CITRULLUS COLOCYNTHIS FRUIT PULP 10 [hp_X] in 29.6 mL DIOSCOREA VILLOSA TUBER (UNII: IWY3IWX2G8) (DIOSCOREA VILLOSA ROOT - UNII:IWY3IWX2G8) DIOSCOREA VILLOSA TUBER 10 [hp_X] in 29.6 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 10 [hp_X] in 29.6 mL SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 10 [hp_X] in 29.6 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:57955-8058-1 1 in 1 CARTON 1 29.6 mL in 1 BOTTLE, SPRAY Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/20/2013 Labeler - King Bio Inc. (617901350) Registrant - King Bio Inc. (617901350) Establishment Name Address ID/FEI Business Operations King Bio Inc. 617901350 api manufacture(57955-8058)