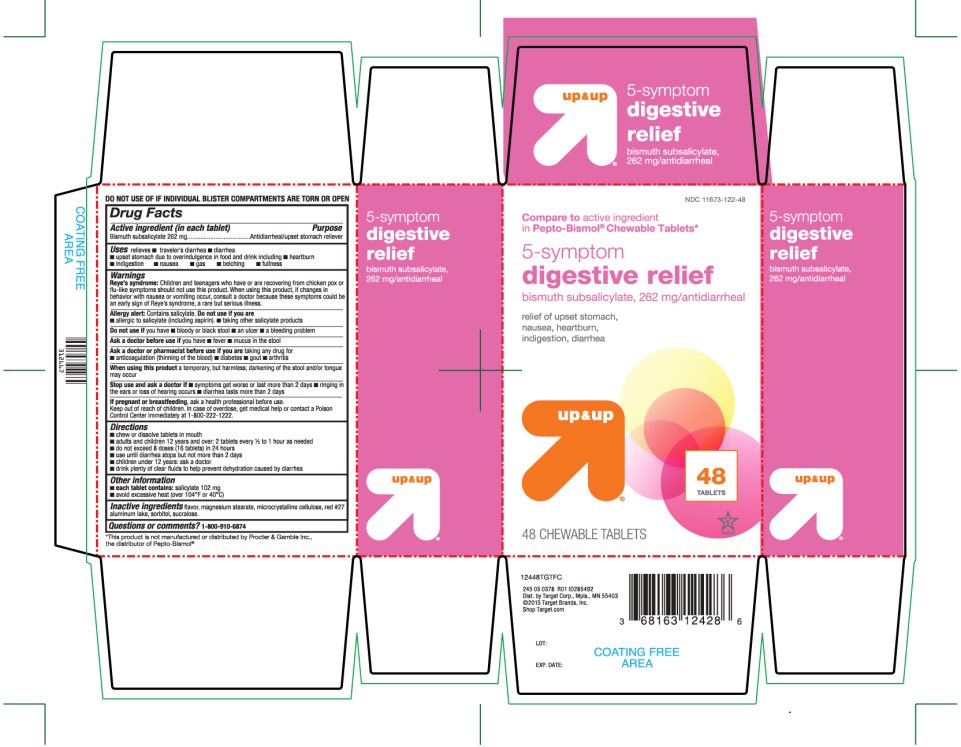
Label: UP AND UP 5-SYMPTOM DIGESTIVE RELIEF- bismuth subsalicylate tablet
- NDC Code(s): 11673-122-48
- Packager: Target Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each tablet)
- Purpose
-
Warnings
Reye's syndrome
Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert:
Contains salicylate. Do not take if you are
- •
- allergic to salicylates (including aspirin)
- •
- taking other salicylate products
Ask a doctor before use if you are taking any drug for
- •
- anticoagulation (thinning of the blood)
- •
- diabetes
- •
- gout
- •
- arthritis
-
Directions
- •
- chew or dissolve tablets in mouth
- •
- adults and children 12 years and older: 2 caplets every 1/2 to 1 hour as needed
- •
- do not exceed 8 doses (16 caplets) in 24 hours
- •
- use until diarrhea stops but not more than 2 days
- •
- children under 12 years: ask a doctor
- •
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- Other information
- Inactive ingredients
-
PRINCIPAL DISPLAY PANEL
NDC 11673-122-48
Compare to active ingredient in Pepto-Bismol® Chewable Tablets*
5- symptom digestive relief
Bismuth subsalicylate, 262 mg/antidiarrheal
relief of upset stomach, nausea, heartburn, indigestion, diarrhea
48 TABLETS
K PARAVE
48 CHEWABLE TABLETS
TAMPER EVIDENT: DO NOT USE IF INDIVIDUAL BLISTER COMPARTMENTS ARE TORN OR OPEN
245 05 0378 R01 ID285492
Dist.by Target Corp., Mpls, MN 55403
©2015 Target Brands, Inc.
Shop Target.com
*This product is not manufactured or distributed by Procter & Gamble Inc., the distributor of PEPTO-BISMOL®
-
INGREDIENTS AND APPEARANCE
UP AND UP 5-SYMPTOM DIGESTIVE RELIEF
bismuth subsalicylate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11673-122 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISMUTH SUBSALICYLATE (UNII: 62TEY51RR1) (BISMUTH CATION - UNII:ZS9CD1I8YE, SALICYLIC ACID - UNII:O414PZ4LPZ) BISMUTH SUBSALICYLATE 262 mg Inactive Ingredients Ingredient Name Strength magnesium stearate (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) D&C RED NO. 27 (UNII: 2LRS185U6K) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color PINK Score no score Shape ROUND Size 16mm Flavor Imprint Code RP124 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11673-122-48 48 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/12/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M008 03/12/2019 Labeler - Target Corporation (006961700)