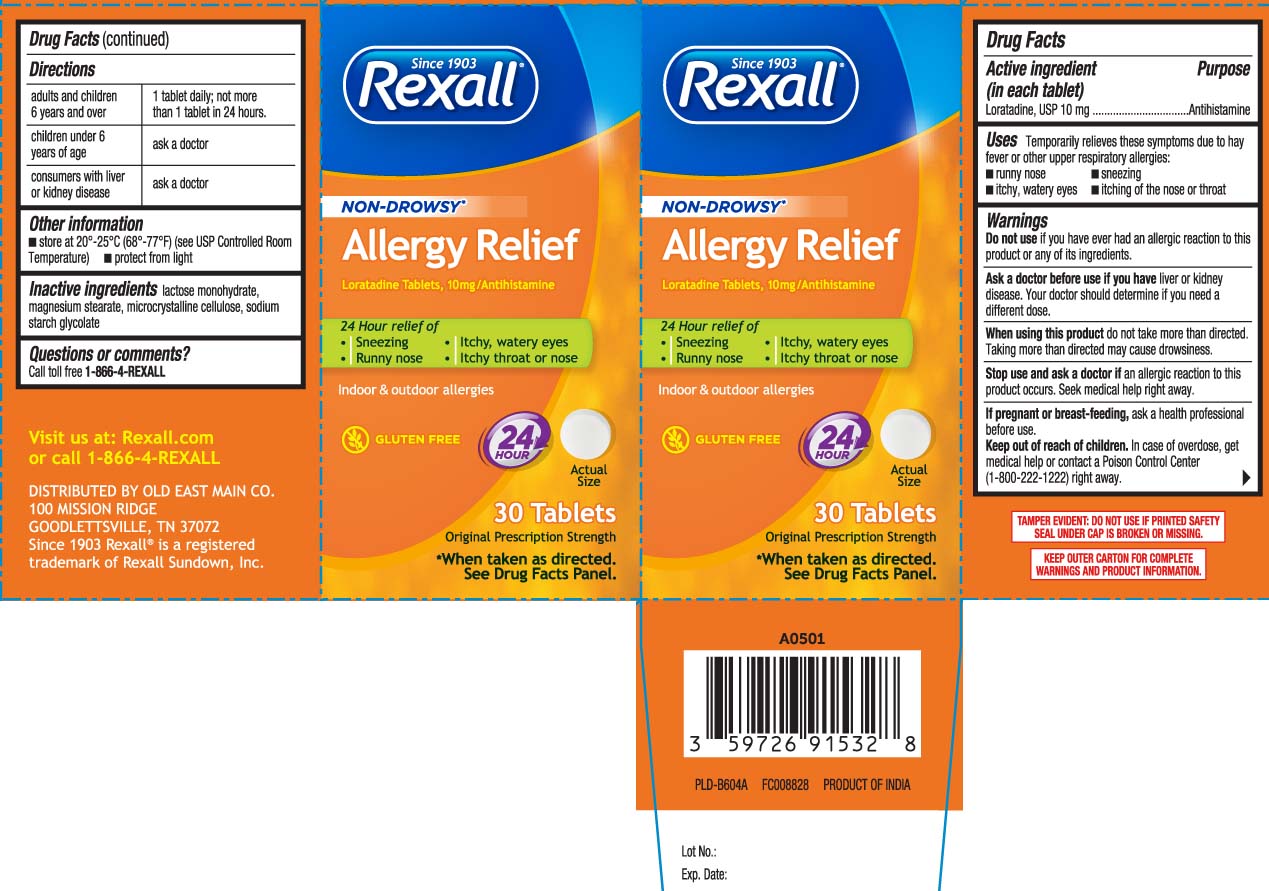
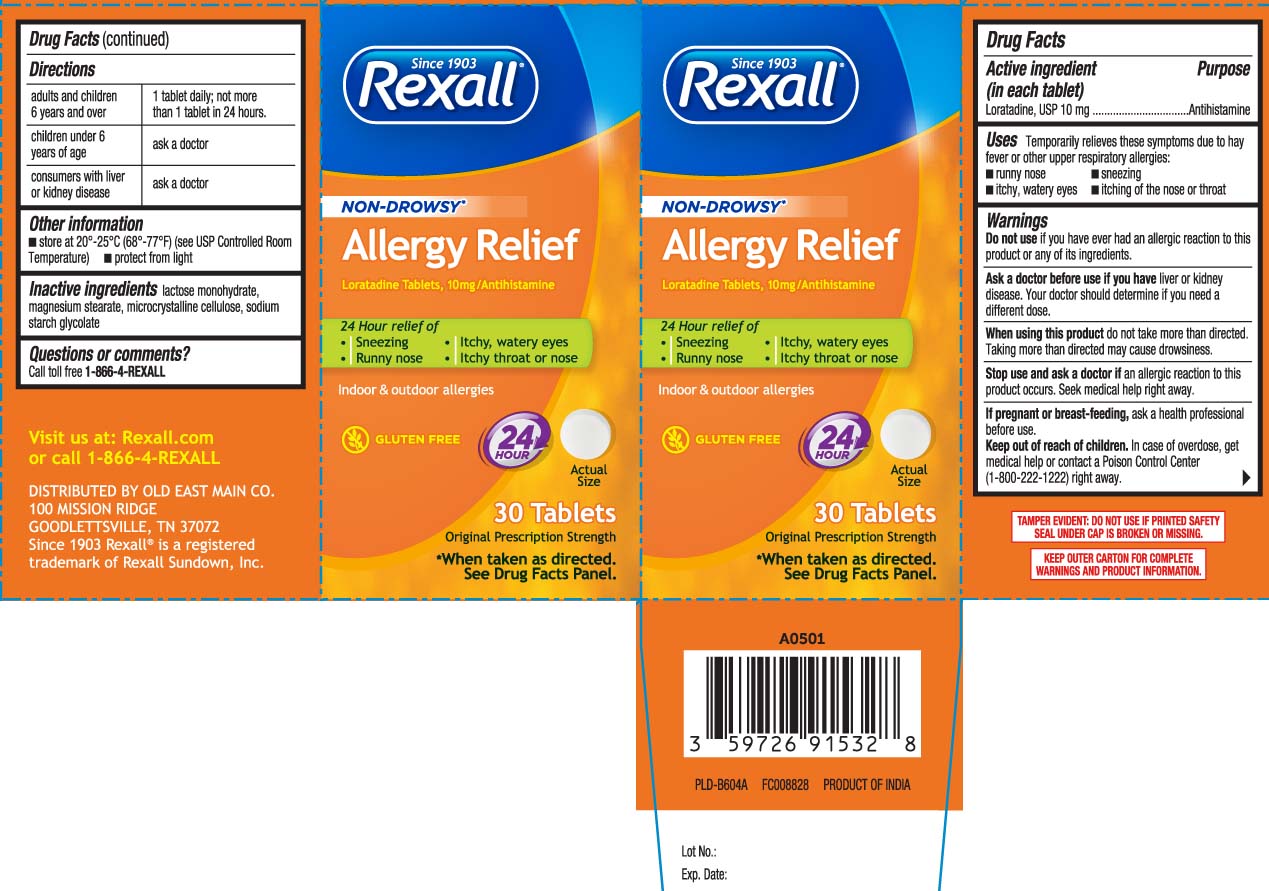
Label: ALL DAY ALLERGY RELIEF- loratadine tablet
- NDC Code(s): 55910-985-30
- Packager: Dolgencorp, Inc. (DOLLAR GENERAL & REXALL)
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 29, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product,
do not take more than directed. Taking more than directed may cause drowsiness.
- Directions
- Other information
- Inactive ingredient
- Questions or comments?
-
Principal Display Panel
NON-DROWSY*
Allergy Relief
Loratadine Tablets, 10 mg/Antihistamine
24-hour relief of
- Sneezing
- Runny nose
- Itchy, watery eyes
- Itchy throat or nose
Indoor & Outdoor allergies
GLUTEN FREE
Original Prescription Strength
*When taken as directed, see drug facts panel.
Tablets
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
DISTRIBUTED BY
OLD EAST MAIN CO.
100 MISSION RIDGE
GOODLETTSVILLE, TN 37072
- Package Label
-
INGREDIENTS AND APPEARANCE
ALL DAY ALLERGY RELIEF
loratadine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55910-985 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) Product Characteristics Color white Score no score Shape ROUND Size 6mm Flavor Imprint Code 439 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55910-985-30 1 in 1 BOX 10/01/2023 1 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075209 10/01/2023 Labeler - Dolgencorp, Inc. (DOLLAR GENERAL & REXALL) (068331990)