Label: SYSTANE COMPLETE PF- propylene glycol emulsion
- NDC Code(s): 0065-1530-01, 0065-1530-04
- Packager: Alcon Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 14, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
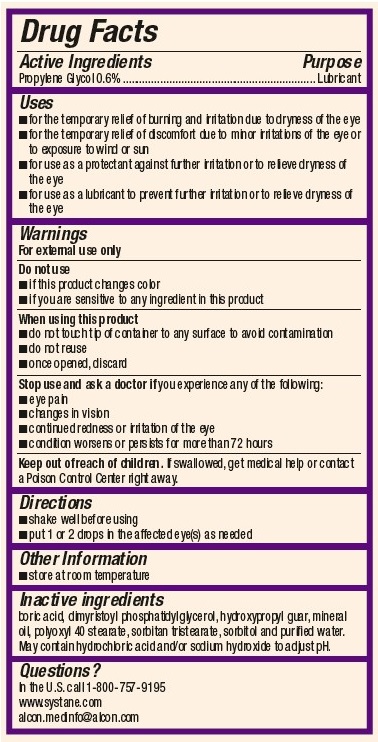
- ACTIVE INGREDIENT
-
Uses
- for the temporary relief of burning and irritation due to dryness of the eye
- for the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun
- for use as a protectant against further irritation or to relieve dryness of the eye
- for use as a lubricant to prevent further irritation or to relieve dryness of the eye
-
Warnings
For external use only
Do not use
- if this product changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- do not touch tip of container to any surface to avoid contamination
- do not reuse
- once opened, discard
- Directions
- Other Information
- Inactive Ingredients
- Questions?
-
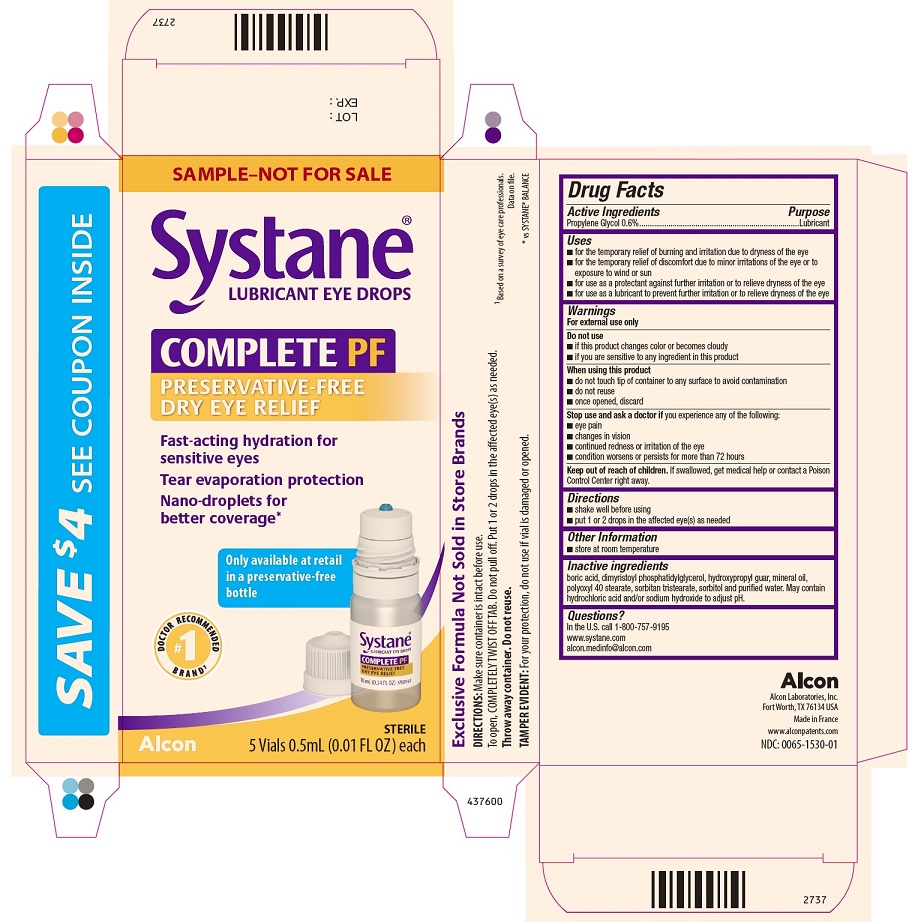
PRINCIPAL DISPLAY PANEL
Alcon Laboratories, Inc.
Fort Worth, TX 76134 USA
Made in France
www.alconpatents.comNDC: 0065-1530-01
437600SAMPLE-NOT FOR SALE
Systane®
LUBRICANT EYE DROP
COMPLETE PF
PRESERVATIVE-FREE
DRY EYE RELIEF
Fast-acting hydration for sensitive eyes
Tear evaporation protection
Nano-droplets for better coverage*
Only available at retail in a preservative-free bottle
#1 DOCTOR RECOMMENDED BRAND1
Alcon
STERILE
5 Vials 0.5 mL (0.01 FL OZ) each
Side Panel:
Exclusive Formula Not Sold in Store Brands
DIRECTIONS: Make sure container is intact before use.
To open, COMPLETELY TWIST OFF TAB. Do not pull off. Put 1 or 2 drops in the affected eye(s) as needed.
Throw away container. Do not reuse.
TAMPER EVIDENT: For your protection, do not use if vial is damaged or opened.
437600
1Based on a survey of eye care professionals.
Data on file.
*vs SYSTANE® BALANCE
LOT :
EXP:
2737

NDC: 0065-1530-04
439260Systane®
LUBRICANT EYE DROPS
COMPLETE PF
PRESERVATIVE-FREE
DRY EYE RELIEF
Convenient
Single Vial
On-the-Go
Fast-acting hydration for sensitive eyes
Tear evaporation protection
DOCTOR RECOMMENDED #1 BRAND1
Alcon
Sterile
30 Vials 0.5mL (0.01 FL OZ) each
Side Panel:
DIRECTIONS:
Make sure container is intact before use.
To open, COMPLETELY TWIST OFF TAB.
Do not pull off.
Put 1 or 2 drops in the affected eye(s) as needed.
Throw away container. Do not reuse.
Alcon
Alcon Laboratories, Inc.
Fort Worth, TX 76134 USA
SYSTANE®COMPLETE
Lubricant Eye Drops
hydrates fast, protects against tear evaporation, and helps support all layers of the tear film
TAMPER EVIDENT: For your protection, this bottle has a tamper evident ring around the bottom of the cap.
Do not use if this ring is damaged or missing at the time of purchase.
1Based on a survey of eye care professionals.
Data on file.
U.S. Pat.:www.alconpatents.com
Made in France
439260-0423
LOT :
EXP.:
439260
-
INGREDIENTS AND APPEARANCE
SYSTANE COMPLETE PF
propylene glycol emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0065-1530 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Propylene Glycol (UNII: 6DC9Q167V3) (Propylene Glycol - UNII:6DC9Q167V3) Propylene Glycol .06 mg in 1 mL Inactive Ingredients Ingredient Name Strength Boric Acid (UNII: R57ZHV85D4) Dimyristoylphosphatidylglycerol, Dl- (UNII: BI71WT9P3R) Guaraprolose (3500 Mpa.S At 1%) (UNII: 3A1I7376TC) Mineral Oil (UNII: T5L8T28FGP) Polyoxyl 40 Stearate (UNII: 13A4J4NH9I) Sorbitan Tristearate (UNII: 6LUM696811) Sorbitol (UNII: 506T60A25R) Water (UNII: 059QF0KO0R) Hydrochloric Acid (UNII: QTT17582CB) Sodium Hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0065-1530-01 5 in 1 CARTON 02/01/2022 1 0.5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product 2 NDC:0065-1530-04 30 in 1 CARTON 12/14/2023 2 0.5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 02/01/2022 Labeler - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Kaysersberg Pharmaceuticals 267486052 manufacture(0065-1530)