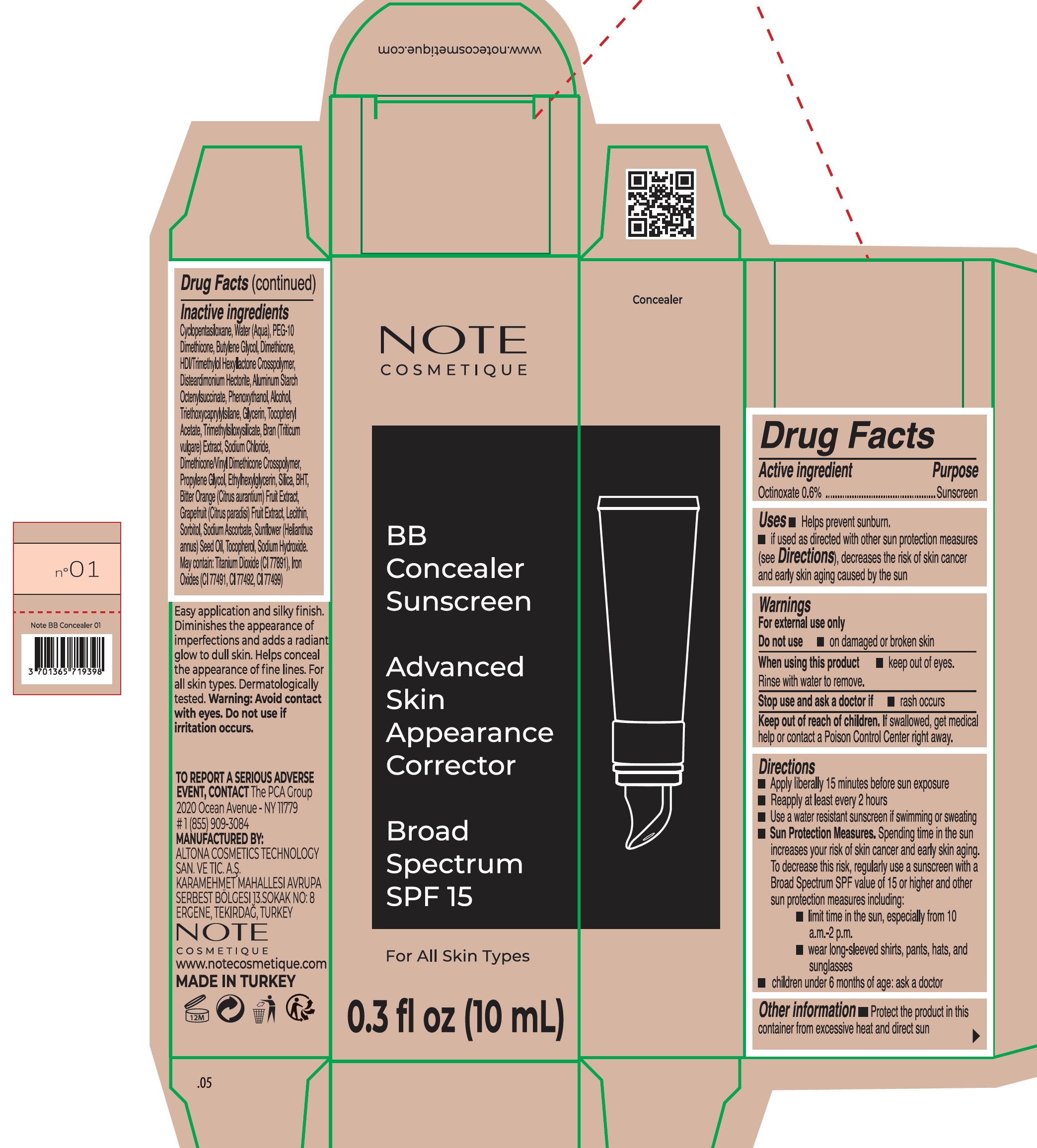
Label: NOTE BB CONCEALER 01- octinoxate cream
- NDC Code(s): 70474-015-01
- Packager: ALTONA COSMETICS TECHNOLOGY SANAYI VE TICARET ANONIM SIRKETI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated September 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
Cyclopentasiloxane, Water (Aqua), PEG-10 Dimethicone, Butylene Glycol, Dimethicone, HDI/Trimethylol Hexyllactone Crosspolymer, Disteardimonium Hectorite, Aluminum Starch Octenylsuccinate, Phenoxyethanol, Alcohol, Triethoxycaprylylsilane, Glycerin, Tocopheryl Acetate, Trimethylsiloxysilicate, Bran (Triticum vulgare) Extract, Sodium Chloride, Dimethicone/Vinyl Dimethicone Crosspolymer, Propylene Glycol, Ethylhexylglycerin, Silica, BHT, Bitter Orange(Citrus aurantium) Fruit Extract, Grapefruit (Citrus paradisi) Fruit Extract, Lecithin, Sorbitol, Sodium Ascorbate, Sunflower (Helianthus annus) Seed Oil, Tocopherol, Sodium Hydroxide. May contain: Titanium Dioxide (CI 77891), Iron Oxides (CI 77491, CI 77492, CI 77499)
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
NOTE BB CONCEALER 01
octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70474-015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6 mg in 1 mL Inactive Ingredients Ingredient Name Strength GRAPEFRUIT (UNII: O82C39RR8C) WHEAT (UNII: 4J2I0SN84Y) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) WATER (UNII: 059QF0KO0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIMETHICONE, UNSPECIFIED (UNII: 92RU3N3Y1O) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) PHENOXYETHANOL (UNII: HIE492ZZ3T) ALCOHOL (UNII: 3K9958V90M) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) GLYCERIN (UNII: PDC6A3C0OX) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SODIUM CHLORIDE (UNII: 451W47IQ8X) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) BITTER ORANGE (UNII: DQD16J2B5O) SORBITOL (UNII: 506T60A25R) SODIUM ASCORBATE (UNII: S033EH8359) SUNFLOWER OIL (UNII: 3W1JG795YI) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70474-015-01 1 in 1 CARTON 01/29/2023 1 10 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/29/2023 Labeler - ALTONA COSMETICS TECHNOLOGY SANAYI VE TICARET ANONIM SIRKETI (502657469) Establishment Name Address ID/FEI Business Operations ALTONA COSMETICS TECHNOLOGY SANAYI VE TICARET ANONIM SIRKETI 502657469 manufacture(70474-015)