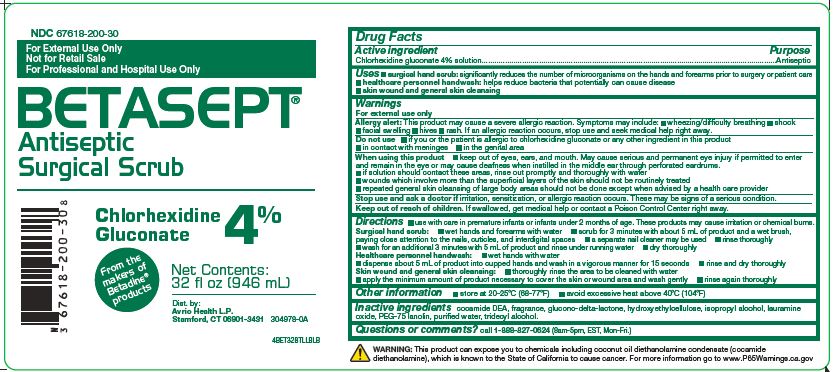
Label: BETASEPT- antiseptic surgical scrub solution
-
NDC Code(s):
67618-200-01,
67618-200-04,
67618-200-08,
67618-200-16, view more67618-200-30, 67618-200-32
- Packager: Atlantis Consumer Healthcare, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
- ACTIVE INGREDIENT
- PURPOSE
- WHEN USING
- WARNINGS
- DO NOT USE
-
WHEN USING
- keep out of eyes, ears, and mouth. May cause serious andpermanent eye injury if permitted to enter and remain in the eye ormay cause deafness when instilled in the middle ear through perforatedeardrums.
- if solution should contact these areas, rinse out promptlyand thoroughly with water
- wounds which involve more than the superficial layers ofthe skin should not be routinely treated
- repeated general skin cleansing of large body areas shouldnot be done except when advised by a health care provider
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- OTHER SAFETY INFORMATION
-
INDICATIONS & USAGE
- wet hands and forearms with water
- scrub for 3 minutes with about 5 mL of product and a wetbrush, paying close attention to the nails, cuticles, and interdigitalspaces
- a separate nail cleaner may be used
- rinse thoroughly
- wash for an additional 3 minutes with 5 mL of product andrinse under running water
- dry thoroughly
- DOSAGE & ADMINISTRATION
- DOSAGE FORMS & STRENGTHS
- OTHER SAFETY INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients cocamide DEA, fragrance, glucono-delta-lactone, hydroxyethylcellulose, isopropyl alcohol, lauramine oxide, PEG-75 lanolin, purified water, tridecyl alcohol
Questions or comments? call 1-888-827-0624 (8am-5pm, EST, Mon-Fri.)
WARNING: This product can expose you to chemicals including coconut oil diethanolamine condensate (cocamide diethanolamine), which is known to the State of California to cause cancer. For more information go to www.P65Warnings.ca.gov
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BETASEPT
antiseptic surgical scrub solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67618-200 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Chlorhexidine Gluconate (UNII: MOR84MUD8E) (Chlorhexidine - UNII:R4KO0DY52L) Chlorhexidine Gluconate .04 mg in 1 mL Inactive Ingredients Ingredient Name Strength COCO DIETHANOLAMIDE (UNII: 92005F972D) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) ISOPROPYL ALCOHOL (UNII: ND2M416302) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) WATER (UNII: 059QF0KO0R) TRIDECYL ALCOHOL (UNII: 8I9428H868) GLUCONOLACTONE (UNII: WQ29KQ9POT) PEG-75 LANOLIN (UNII: 09179OX7TB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67618-200-04 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/1993 2 NDC:67618-200-08 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/1993 3 NDC:67618-200-16 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/1993 4 NDC:67618-200-30 946 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/1993 5 NDC:67618-200-32 946 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/1993 01/31/2023 6 NDC:67618-200-01 3785 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/01/1993 05/31/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019125 06/01/1993 Labeler - Atlantis Consumer Healthcare, Inc. (118983925) Registrant - Atlantis Consumer Healthcare, Inc. (118983925)