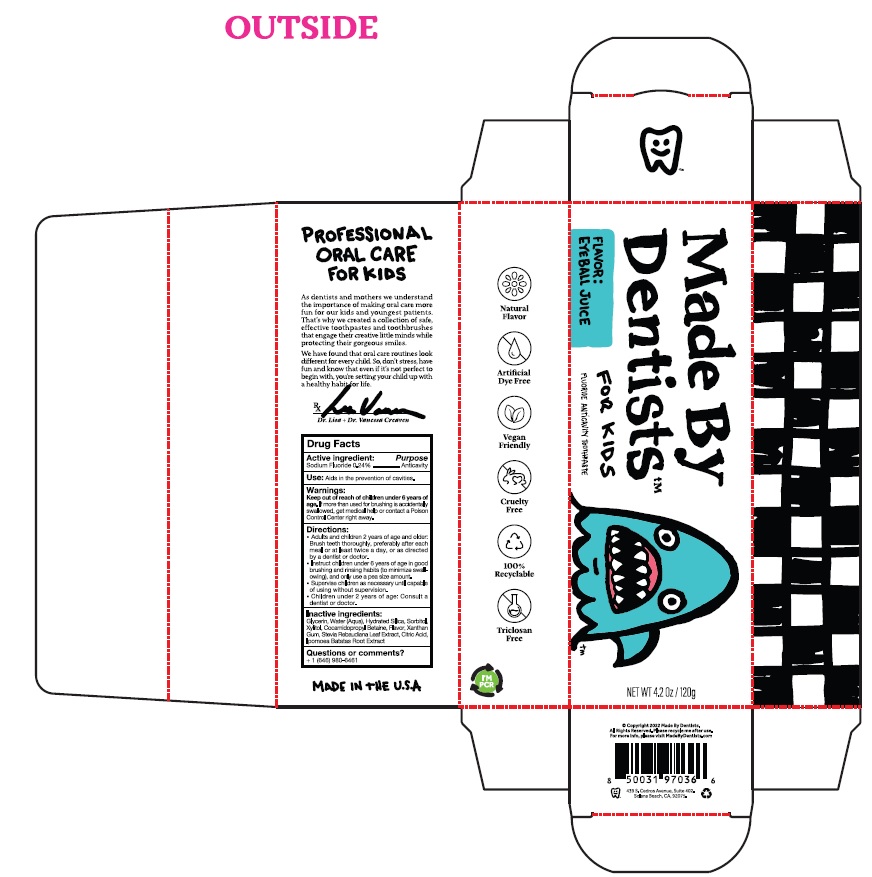
Label: MADE BY DENTISTS, INC. EYEBALL JUICE- sodium fluoride paste
- NDC Code(s): 75065-022-06
- Packager: Made By Dentists, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions:
• Adults and children 2 years of age and older:
Brush teeth thoroughly, preferably after each
meal or at least twice a day, or as directed
by a dentist or doctor.
• Instruct children under 6 years of age in good
Brushing and rinsing habits (to minimize swall-
owing), and only use a pea size amount
• Supervise children as necessary until capable
of using without supervision.
• Children under 2 years of age: Consult a
dentist or doctor. - INACTIVE INGREDIENT
- QUESTIONS
- EyeBall Toothpaste Product Labeling
-
INGREDIENTS AND APPEARANCE
MADE BY DENTISTS, INC. EYEBALL JUICE
sodium fluoride pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75065-022 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 0.24 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) HYDRATED SILICA (UNII: Y6O7T4G8P9) SORBITOL (UNII: 506T60A25R) XYLITOL (UNII: VCQ006KQ1E) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) XANTHAN GUM (UNII: TTV12P4NEE) STEVIA REBAUDIUNA LEAF (UNII: 6TC6NN0876) CITRIC ACID ACETATE (UNII: DSO12WL7AU) SWEET POTATO (UNII: M9WGG9Z9GK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75065-022-06 1 in 1 CARTON 01/24/2023 1 120 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M021 01/24/2023 Labeler - Made By Dentists, Inc. (117405870) Registrant - Made By Dentists, Inc. (117405870)