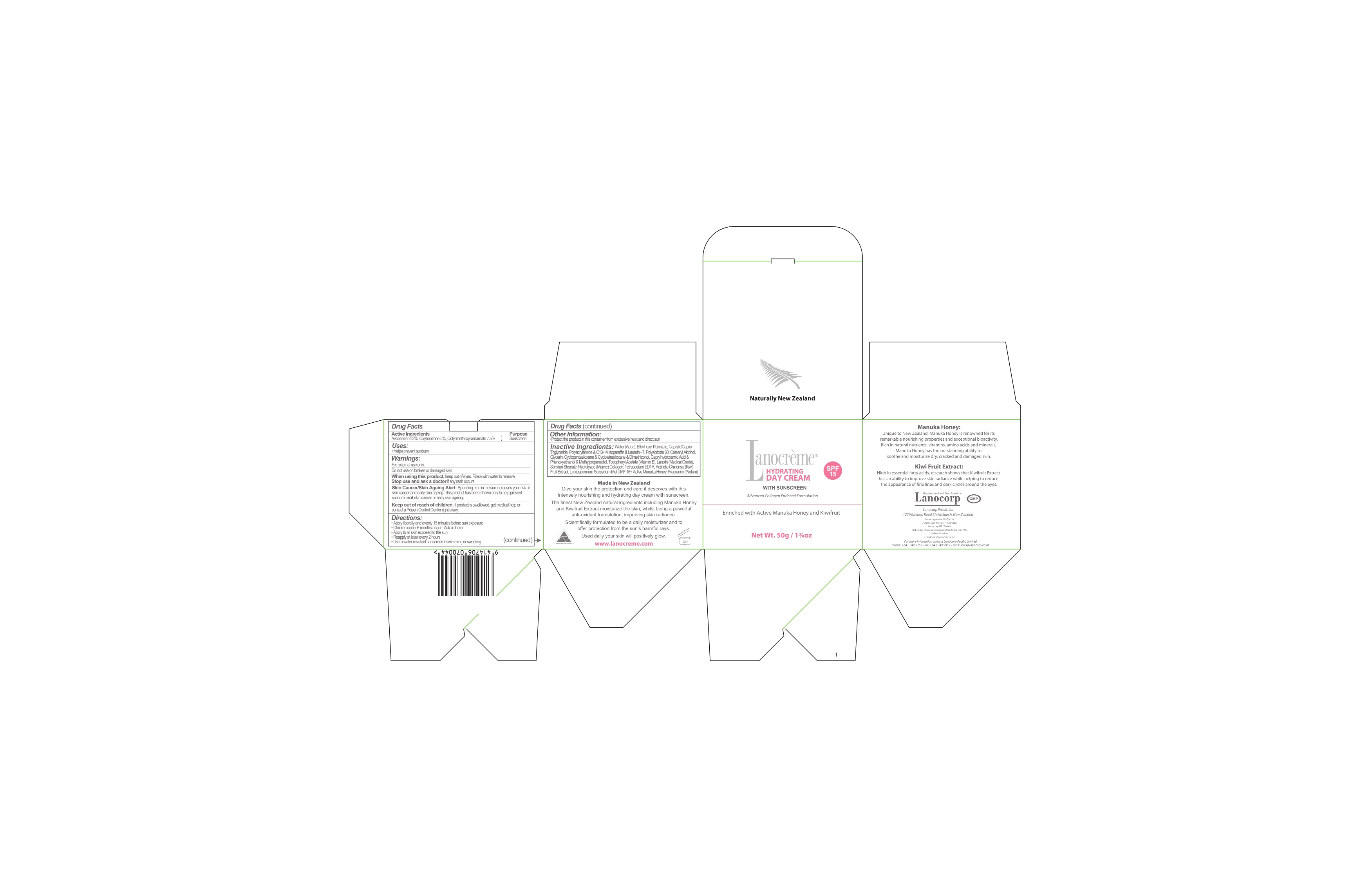
Label: LANOCREME HYDRATING DAY SPF15- avobenzone, oxybenzone, octyl methoxycinnamate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 43617-3415-1 - Packager: Lanocorp Pacific Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 27, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
-
WARNINGS
WARNINGS
For external use only. Do not use on broken or damaged skin.
Skin Cancer/Skin Ageing Alert: Spending time in the sun increases your risk of skin cancer and early skin ageing. This product has been shown only to help prevent sunburn, not skin cancer or early skin ageing.
-
INACTIVE INGREDIENT
INACTIVE INGREDIENTS
Water
Ethylhexyl palmitate
Caprylic triglyceride
Capric triglyceride
Polyacrylamide
c13-14 Isoparaffin
Laureth-7
Polysorbate 60
Cetearyl alcohol
Glycerin
Cyclopentasiloxane
Clyclotetrasiloxane
dimethiconol
Caprylhydroxamic acid
phenoxyethanol
methylpropanediol
tocopheryl acetate (vitamin e)
lanolin (medical grade)
Sorbitan stearate
Hydrolyzed (Marine) collagen
Tetrasodium EDTA
Actinidia chinensis (kiwi) fruit extract
Leptospermum scoparium Mel UMF 15+ Active Manuka Honey
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LANOCREME HYDRATING DAY SPF15
avobenzone, oxybenzone, octyl methoxycinnamate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43617-3415 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1.5 g in 50 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 1.5 g in 50 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 50 g Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Ethylhexyl palmitate (UNII: 2865993309) Tricaprylin (UNII: 6P92858988) Tricaprin (UNII: O1PB8EU98M) SODIUM ACRYLOYLDIMETHYLTAURATE-ACRYLAMIDE COPOLYMER (1:1; 90000-150000 MPA.S) (UNII: 5F4963KLHS) C13-14 Isoparaffin (UNII: E4F12ROE70) Laureth-7 (UNII: Z95S6G8201) Polysorbate 60 (UNII: CAL22UVI4M) Cetostearyl Alcohol (UNII: 2DMT128M1S) Glycerin (UNII: PDC6A3C0OX) Cyclomethicone 5 (UNII: 0THT5PCI0R) Cyclomethicone 4 (UNII: CZ227117JE) Dimethiconol (41 MPA.S) (UNII: 343C7U75XW) Caprylhydroxamic acid (UNII: UPY805K99W) Phenoxyethanol (UNII: HIE492ZZ3T) Methylpropanediol (UNII: N8F53B3R4R) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Lanolin (UNII: 7EV65EAW6H) Sorbitan monostearate (UNII: NVZ4I0H58X) HYDROLYSED MARINE COLLAGEN (ENZYMATIC; 2000 MW) (UNII: 2WID9OCG7P) EDETATE SODIUM (UNII: MP1J8420LU) KIWI FRUIT (UNII: 71ES77LGJC) HONEY (UNII: Y9H1V576FH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43617-3415-1 1 in 1 BOX 1 50 g in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 04/30/2012 Labeler - Lanocorp Pacific Ltd (594482114) Establishment Name Address ID/FEI Business Operations Lanocorp Pacific Ltd 594482114 manufacture