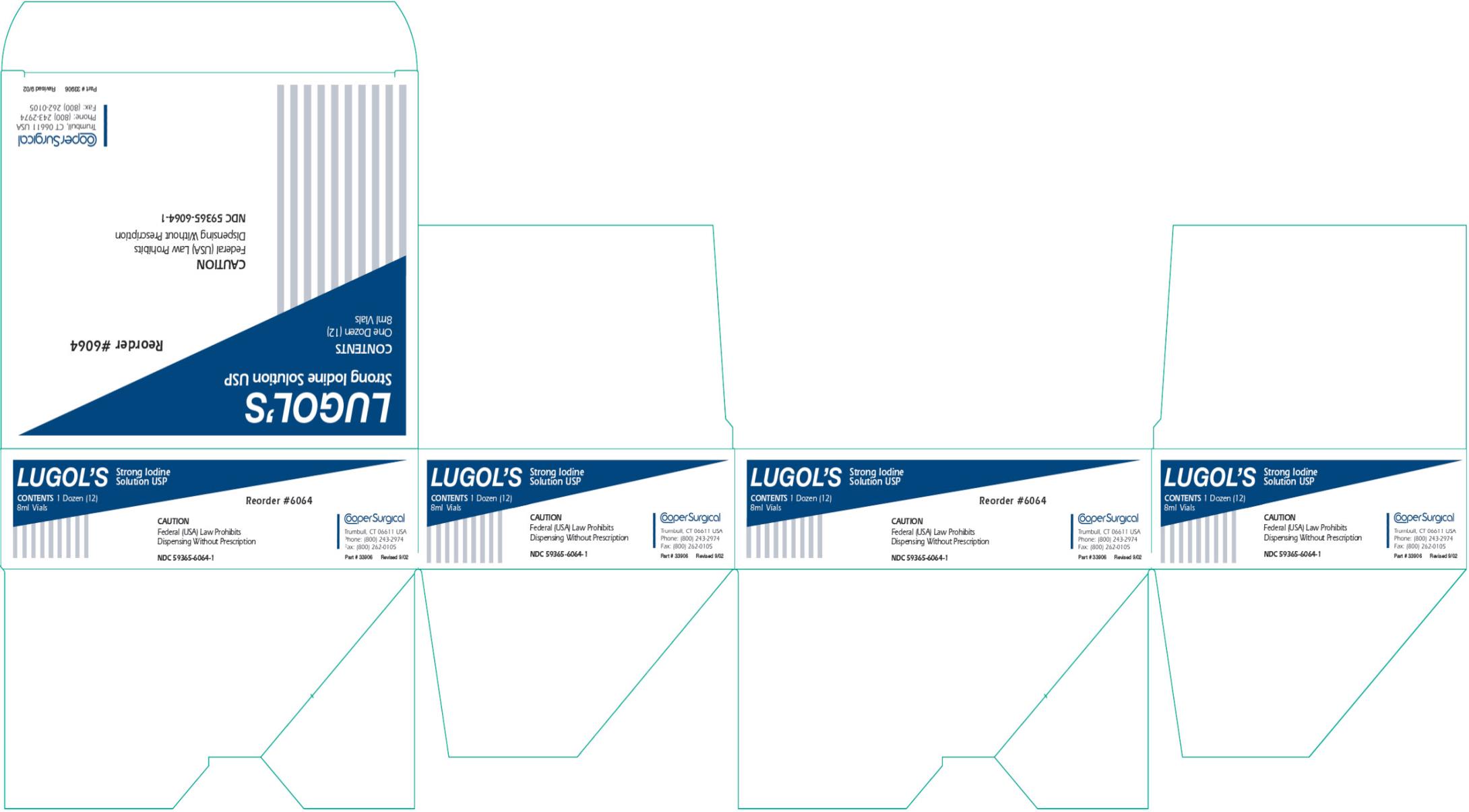
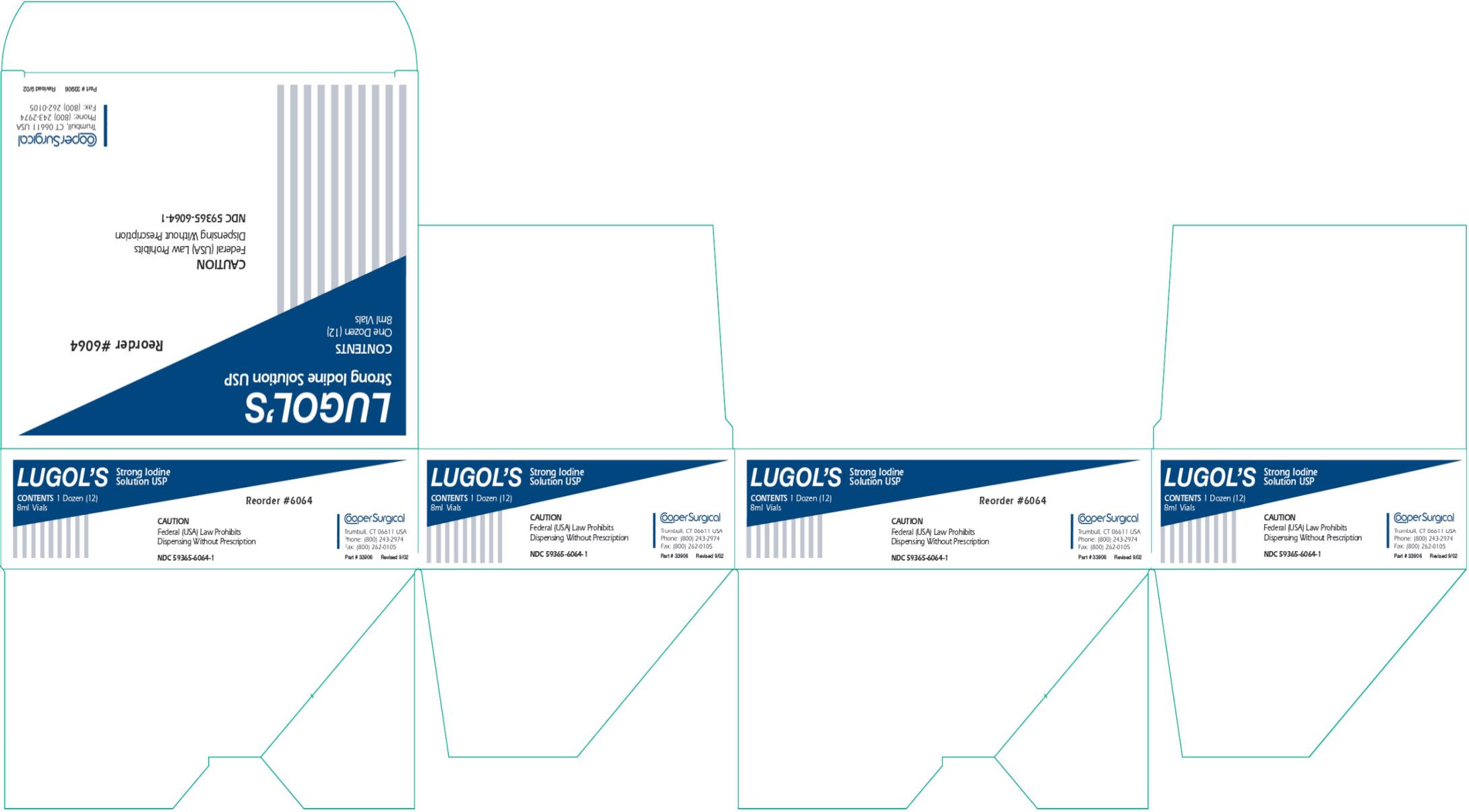
Label: LUGOLS STRONG IODINE- iodine and potassium iodide solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 59365-6064-0, 59365-6064-1 - Packager: CooperSurgical, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 25, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CONTENTS
- DESCRIPTION
- HOW SUPPLIED
- INDICATIONS AND USAGE
- ADMINISTRATION
- WARNINGS
- CONTRAINDICATIONS
- CAUTION
- STORAGE
-
DISPOSAL
Opened containers with unused portions of product and applicator swabs containing residual product should be placed in a suitable, dry container for disposal following local hazardous waste practices. Waste containing LUGOL’S should not be subjected to any thermal process whether intended for destruction or recycling purposes.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LUGOLS STRONG IODINE
iodine and potassium iodide solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59365-6064 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 0.05 g in 1 mL POTASSIUM IODIDE (UNII: 1C4QK22F9J) (IODIDE ION - UNII:09G4I6V86Q) POTASSIUM IODIDE 0.100 g in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59365-6064-1 12 in 1 CARTON 10/01/1992 1 NDC:59365-6064-0 8 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 10/01/1992 Labeler - CooperSurgical, Inc. (801895244)