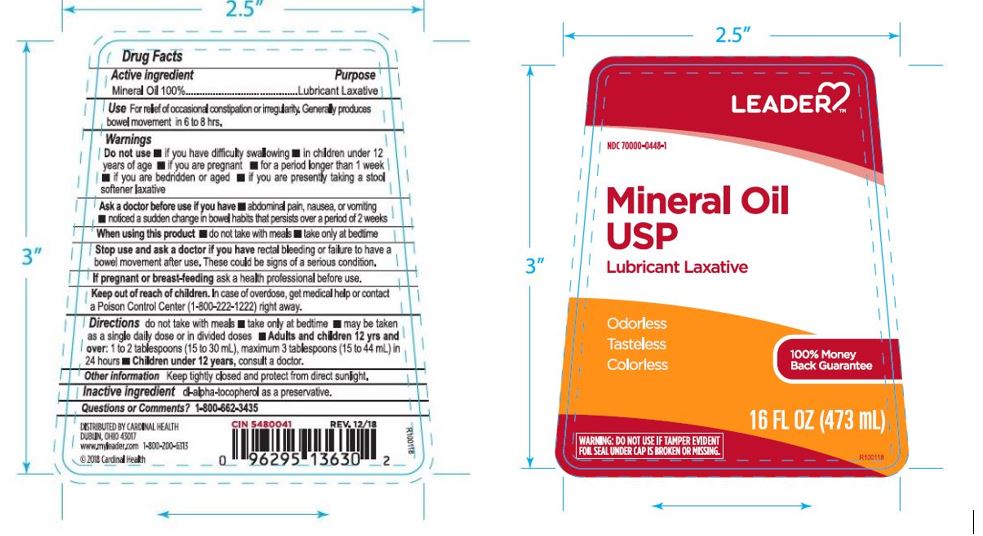
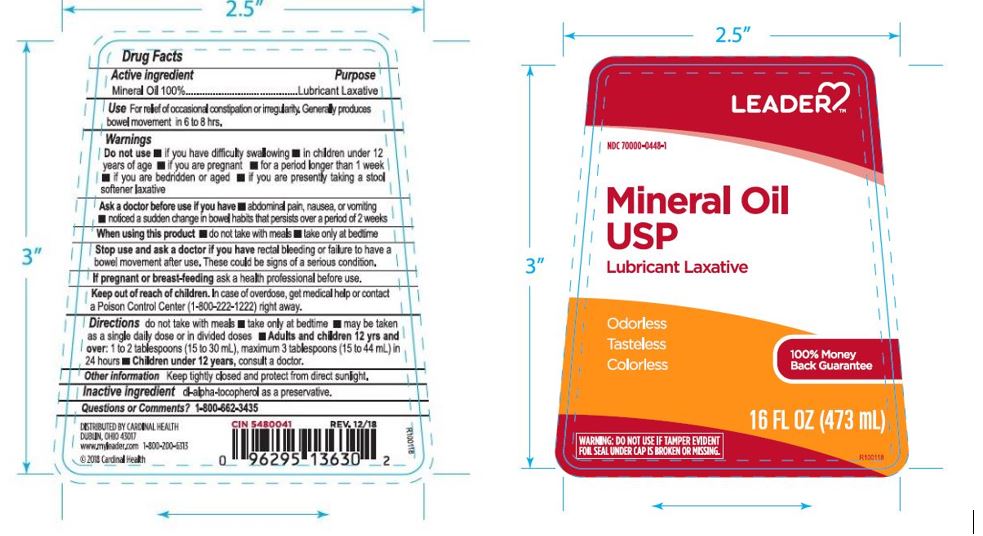
Label: LEADER MINERAL OIL- mineral oil liquid
- NDC Code(s): 70000-0448-1
- Packager: Cardinal Health, 110 dba Leader
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 22, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LEADER MINERAL OIL
mineral oil liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70000-0448 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINERAL OIL (UNII: T5L8T28FGP) (MINERAL OIL - UNII:T5L8T28FGP) MINERAL OIL 1000 mg in 1 mL Inactive Ingredients Ingredient Name Strength .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70000-0448-1 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/18/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 12/18/2018 Labeler - Cardinal Health, 110 dba Leader (063997360) Registrant - Pharma Nobis, LLC (118564114) Establishment Name Address ID/FEI Business Operations Pharma Nobis, LLC 118564114 analysis(70000-0448) , manufacture(70000-0448) , pack(70000-0448) , label(70000-0448)