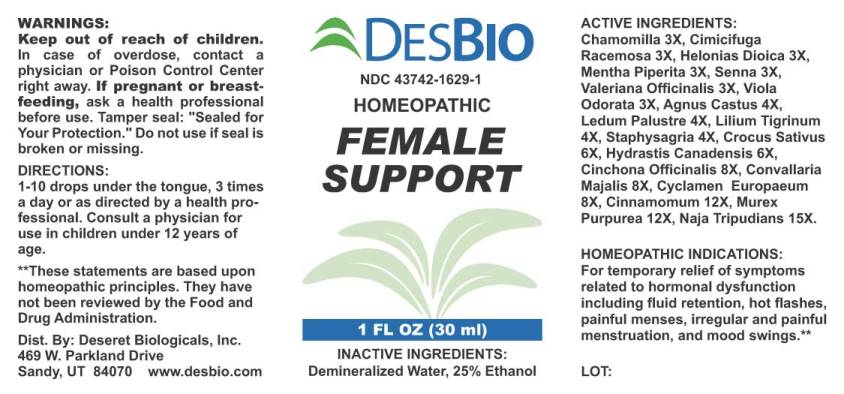
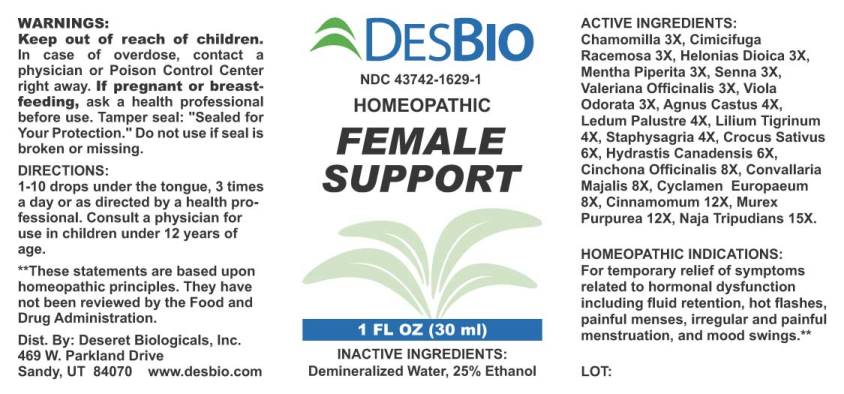
Label: FEMALE SUPPORT (chamomilla, cimicifuga racemosa, helonias dioica, mentha piperita, senna- cassia angustifolia, valeriana officinalis, viola odorata, agnus castus, ledum palustre, lilium tigrinum, staphysagria, crocus sativus, hydrastis canadensis, cinchona officinalis, convallaria majalis, cyclamen europaeum, cinnamomum, murex purpurea, naja tripudians liquid
- NDC Code(s): 43742-1629-1
- Packager: Deseret Biologicals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated June 28, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS:
Chamomilla 3X, Cimicifuga Racemosa 3X, Helonias Dioica 3X, Mentha Piperita 3X, Senna (Cassia Angustifolia) 3X, Valeriana Officinalis 3X, Viola Odorata 3X, Agnus Castus 4X, Ledum Palustre 4X, Lilium Tigrinum 4X, Staphysagria 4X, Crocus Sativus 6X, Hydrastis Canadensis 6X, Cinchona Officinalis 8X, Convallaria Majalis 8X, Cyclamen Europaeum 8X, Cinnamomum 12X, Murex Purpurea 12X, Naja Tripudians 15X.
- HOMEOPATHIC INDICATIONS:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- HOMEOPATHIC INDICATIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
FEMALE SUPPORT
chamomilla, cimicifuga racemosa, helonias dioica, mentha piperita, senna (cassia angustifolia), valeriana officinalis, viola odorata, agnus castus, ledum palustre, lilium tigrinum, staphysagria, crocus sativus, hydrastis canadensis, cinchona officinalis, convallaria majalis, cyclamen europaeum, cinnamomum, murex purpurea, naja tripudians liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:43742-1629 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) (MATRICARIA CHAMOMILLA - UNII:G0R4UBI2ZZ) MATRICARIA RECUTITA 3 [hp_X] in 1 mL BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 3 [hp_X] in 1 mL CHAMAELIRIUM LUTEUM ROOT (UNII: DQV54Y5H3U) (CHAMAELIRIUM LUTEUM ROOT - UNII:DQV54Y5H3U) CHAMAELIRIUM LUTEUM ROOT 3 [hp_X] in 1 mL MENTHA PIPERITA (UNII: 79M2M2UDA9) (MENTHA PIPERITA - UNII:79M2M2UDA9) MENTHA PIPERITA 3 [hp_X] in 1 mL SENNA LEAF (UNII: AK7JF626KX) (SENNA LEAF - UNII:AK7JF626KX) SENNA LEAF 3 [hp_X] in 1 mL VALERIAN (UNII: JWF5YAW3QW) (VALERIAN - UNII:JWF5YAW3QW) VALERIAN 3 [hp_X] in 1 mL VIOLA ODORATA (UNII: AET12U8B74) (VIOLA ODORATA - UNII:AET12U8B74) VIOLA ODORATA 3 [hp_X] in 1 mL CHASTE TREE FRUIT (UNII: 433OSF3U8A) (CHASTE TREE - UNII:433OSF3U8A) CHASTE TREE FRUIT 4 [hp_X] in 1 mL LEDUM PALUSTRE TWIG (UNII: 877L01IZ0P) (LEDUM PALUSTRE TWIG - UNII:877L01IZ0P) LEDUM PALUSTRE TWIG 4 [hp_X] in 1 mL LILIUM LANCIFOLIUM WHOLE FLOWERING (UNII: X67Z2963PI) (LILIUM LANCIFOLIUM WHOLE FLOWERING - UNII:X67Z2963PI) LILIUM LANCIFOLIUM WHOLE FLOWERING 4 [hp_X] in 1 mL DELPHINIUM STAPHISAGRIA SEED (UNII: 00543AP1JV) (DELPHINIUM STAPHISAGRIA SEED - UNII:00543AP1JV) DELPHINIUM STAPHISAGRIA SEED 4 [hp_X] in 1 mL SAFFRON (UNII: E849G4X5YJ) (SAFFRON - UNII:E849G4X5YJ) SAFFRON 6 [hp_X] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 6 [hp_X] in 1 mL CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 8 [hp_X] in 1 mL CONVALLARIA MAJALIS (UNII: QHH4HVF5QE) (CONVALLARIA MAJALIS - UNII:QHH4HVF5QE) CONVALLARIA MAJALIS 8 [hp_X] in 1 mL CYCLAMEN PURPURASCENS TUBER (UNII: G728143D8Q) (CYCLAMEN PURPURASCENS TUBER - UNII:G728143D8Q) CYCLAMEN PURPURASCENS TUBER 8 [hp_X] in 1 mL CINNAMON (UNII: 5S29HWU6QB) (CINNAMON - UNII:5S29HWU6QB) CINNAMON 12 [hp_X] in 1 mL HEXAPLEX TRUNCULUS HYPOBRANCHIAL GLAND JUICE (UNII: IQV54TN60Y) (HEXAPLEX TRUNCULUS HYPOBRANCHIAL GLAND JUICE - UNII:IQV54TN60Y) HEXAPLEX TRUNCULUS HYPOBRANCHIAL GLAND JUICE 12 [hp_X] in 1 mL NAJA NAJA VENOM (UNII: ZZ4AG7L7VM) (NAJA NAJA VENOM - UNII:ZZ4AG7L7VM) NAJA NAJA VENOM 15 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43742-1629-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 03/18/2020 04/22/2026 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 03/18/2020 04/22/2026 Labeler - Deseret Biologicals, Inc. (940741853) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(43742-1629) , api manufacture(43742-1629) , label(43742-1629) , pack(43742-1629)