Label: ADSOL RED CELL PRESERVATION SOLUTION SYSTEM IN PLASTIC CONTAINER (PL 146 PLASTIC) (anticoagulant citrate phosphate dextrose- cpd solution and adsol preservation solution kit
- NDC Code(s): 0942-6461-02
- Packager: Fenwal, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 25, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
4R3440, 4R3468, 4R3464
Fresenius Kabi
Fenwal Blood-Pack Units Rx only
Using Anticoagulant Citrate Phosphate Dextrose Solution, USP (CPD) with an Integrally Attached Container of Adsol Red Cell Preservation Solution and Fenwal HighFlo Needle
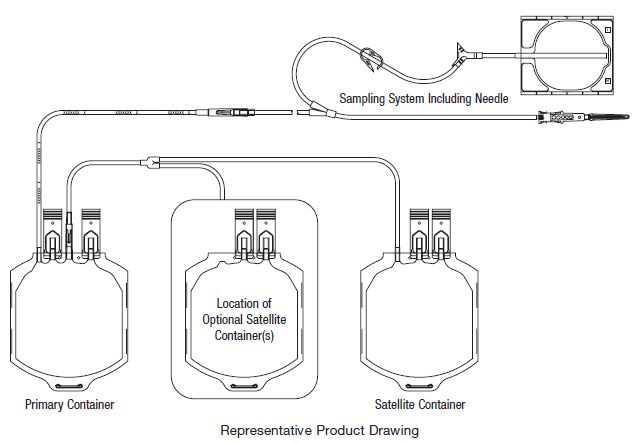
Contains Sample Diversion System for the collection of whole blood samples for laboratory testing.
Instructions for Use
Collection Procedure:
Use aseptic technique.
Notes:
- • If Sample Diversion System is not used, donor samples may be collected using an alternate method following standard procedures.
- • Nominal tubing dimensions of product are 0.118" inner diameter x 0.025" wall thickness.
Precautions:
- • Upon removal of Blood-Pack unit from the clear plastic overwrap, visually inspect the unit.
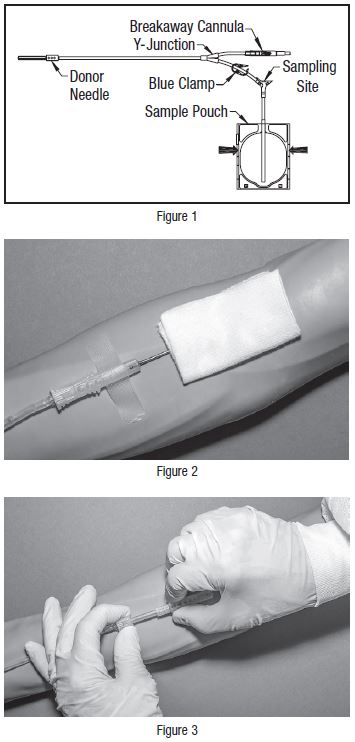
- • Do not use the product if the in-line cannula is broken and/or anticoagulant is present in the sample pouch or in the tubing from the in-line cannula to the sample pouch and donor needle (see Figure 1). Note that condensation in the empty tubing of the Blood-Pack unit is expected as a result of the sterilization process.
- • Do not use unless the solutions are clear.
1. Identify Blood-Pack unit using appropriate donor identification system.
2. Donor Scale
- • Adjust donor scale to desired collection weight.
- • Position primary container on the donor scale as far as possible below donor arm.
3. Clamp donor tubing between the HighFlo1 needle and primary container with clamp. (This step can be performed prior to step 1 or 2.)
4. Visually inspect the tubing from the in-line cannula to the sample pouch and donor needle, as well as the sample pouch to reconfirm that there is no anticoagulant present.
Note: Ensure that the sample pouch remains below the donor’s arm.
5. Following blood center procedures, apply pressure to donor’s arm and disinfect site of
venipuncture.
6. Remove needle cover per instructions below:
- • Holding the hub and cover near the tamper-evident seal, twist cover and hub in opposite directions to break seal.
- • Remove needle cover, being careful not to drag the cover across the needle point.
7. Following blood center procedures, perform venipuncture, appropriately secure donor needle and/or tubing and release hemostat.
8. When good blood flow is established, stabilize the front of the needle guard to arm with tape (see Figure 2).
9. Allow the sample pouch to fill with blood according to center procedure. Monitor blood flow into sample pouch.
Notes:
- • The sample pouch contains an average fill volume of approximately 53 mL with a maximum fill volume of approximately 60 mL when filled to capacity.
- • If less blood sample volume is required, the flow to the sample pouch may be stopped prior to completely filling the pouch. For example, in order to target a fill volume of approximately 40 mL, fill to the level indicated by the arrows in Figure 1. Ensure the pouch is hanging vertically.
- • The tube leading from the Y-junction to the sample pouch contains an additional volume of approximately 2 mL.
Precautions:
- • Do not elevate or squeeze the sample pouch as this could cause blood to backflow from the sample pouch into the collection system.
- • Once the sample pouch is filled to desired volume, complete steps 10 – 18 within approximately 4 minutes to avoid possible clot formation in the tubing and/or sample pouch.
10. Close the blue clamp on tubing between the Y-junction and the sample pouch.
11. Break the in-line cannula below the Y-junction in the donor tubing to the primary container allowing blood collection to proceed. To completely break the in-line cannula, grasp with both hands. Snap it at a 90° angle in one direction, and then bend it at a 90° angle in the opposite direction. Ensure the in-line cannula is completely broken and that the blood flows freely to the primary container.
Precaution: Failure to break the in-line cannula completely may result in restricted blood flow.
12. Following blood center procedures, mix blood and anticoagulant in the primary container immediately and at several intervals during collection.
13. Following blood center procedures, hermetically seal the tubing between the sampling site and the Y-junction to maintain sterility of the blood collection system prior to removing blood samples.
Warning:
- • Do not proceed with the remaining steps until the tubing leading to the sample pouch is hermetically sealed between the sampling site and the Y-junction. To maintain the whole blood collection container as a closed system, the tubing between the sample pouch and Y-junction must be hermetically sealed prior to inserting the access device into the sampling site. Failure to do so may lead to contamination of the whole blood collection.
14. Insert the access device by pushing firmly into the sampling site until the membrane seal is penetrated.
Note: If the access device is assembled such that the outer barrel is screwed onto the Luer, make sure to rotate clockwise upon insertion to avoid barrel detaching from Luer.
15. Open the cap on the access device (if applicable). Hold access device so that the
sample pouch hangs down.
16. Directly align the vacuum sample tube with the internal needle in the access device. Insert vacuum sample tube into device.
17. Allow vacuum sample tube to fill with blood then remove from the access device.
18. Repeat steps 16 and 17 until the desired number of vacuum sample tubes have been filled.
Notes:
- • If the access device needs to be replaced, clamp the tubing between the sampling site and the sample pouch. Then, grasp base of sampling site with one hand and pull the access device out with the other hand. Firmly insert the new access device. Remove clamp and continue sampling.
- • If the access device is assembled such that the outer barrel is screwed onto the Luer, make sure to rotate clockwise upon removal to avoid barrel detaching from Luer.
- • The access device can only be replaced one time.
Precaution: When replacing access device, be careful to avoid contact with any blood droplets on the Luer or sampling site. Discard used access device appropriately.
19. Collect the appropriate volume based on Blood-Pack unit used.
Note: The volume of anticoagulant is sufficient for the blood collection indicated on Blood-Pack unit ± 10%.
Precaution: Once the desired blood volume is collected, complete steps 20-23 within approximately 4 minutes to avoid possible clot formation in the tubing.
20. Release pressure on the donor’s arm. If appropriate, apply clamp to donor tubing
between the needle and the Y-junction.
21. Hermetically seal donor tubing near in-line cannula on side leading to the primary
container.
22. Withdrawal of Needle (see Figure 3)
Precaution: The needle guard must be held stationary while the needle is withdrawn into it.
- a)
- Place folded sterile gauze over puncture site and hold in place with finger tip without exerting pressure.
- b)
- Hold sides of needle guard near the front, between the index finger and thumb. Pull the hub back smoothly until the needle is completely enclosed and securely locked into the needle guard.
- c)
- Confirm the needle is completely enclosed and securely locked into the needle guard.
23. Strip blood from donor tubing into primary container, mix and allow the tubing to refill; repeat once.
24. Seal at X marks on donor tubing to provide numbered aliquots of anticoagulated
blood for typing or crossmatching.
Note: Step 25 may be performed prior to step 23 or 24 if desired.
25. Remove and discard the Sample Diversion System and the donor needle in the
needle guard into an appropriate biohazardous waste container following established
procedures.
Component Preparation:
Notes:
- • If a platelet concentrate is to be prepared, it should be separated from the red blood cells within 8 hours after blood collection.
- • Fresh frozen plasma should be separated from the red blood cells and placed in the freezer at -18°C or colder within 8 hours after blood collection.
- • Adsol red cell preservation solution should be added to the red blood cells immediately after the removal of plasma. Preparation of AS-1 Red Blood Cells may vary depending on processing option selected:
- a) Within 8 hours of blood collection if whole blood is held at ambient temperature.
- b) Within 3 days of blood collection if whole blood is refrigerated.
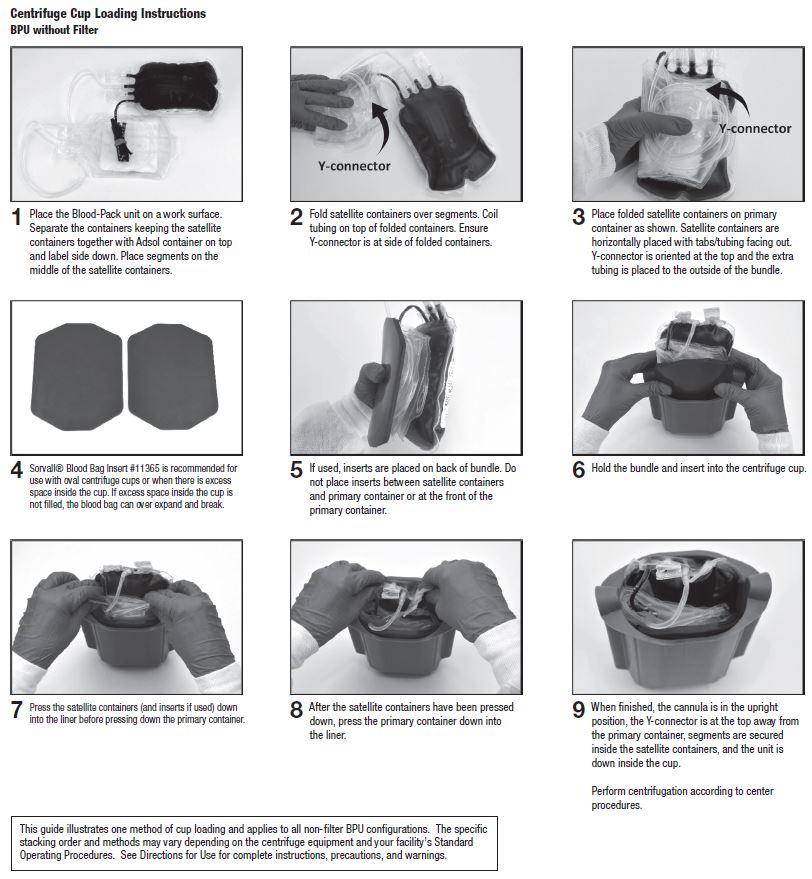
26. At the appropriate time, prepare the Blood-Pack unit for centrifugation by thoroughly mixing the primary container end over end, then load the unit in a centrifuge cup per the instructions on page 3.
27. Following centrifugation, remove containers from the centrifugation cup taking care not to disturb the red blood cell / plasma interface.
28. Place primary container in plasma extractor and express plasma into empty Transfer Pack container by releasing pressure plate and opening closure in tubing of primary container.
29. When desired amount of plasma has been removed, clamp tubing between Y and
plasma container.
30. Suspend Adsol red cell preservation solution container, open closure in tubing and
drain contents into primary container of CPD red blood cells. Clamp tubing.
31. Seal transfer tubing in three places between the Y-connector and primary container.
Cut middle seal being careful to avoid fluid splatter. For Double Blood-Pack unit codes, discard Adsol solution container. For other Adsol codes, the empty solution container may be used as a Transfer Pack container for further component
preparation.
32. Mix Adsol red cell preservation solution and red cells thoroughly.
33. Store suspended AS-1 Red Blood Cells between 1 and 6°C.
34. Infuse AS-1 Red Blood Cells within 42 days of collection.
Warning: Failure to achieve closed system processing conditions negates the extended storage claim and the red blood cell product must be transfused within 24 hours.
Store at Controlled Room Temperature. Protect from freezing. Avoid excessive heat.
Definition of “Controlled Room Temperature”:
“A temperature maintained thermostatically that encompasses the usual and customary working environment of 20° to 25°C (68° to 77°F); that results in a mean kinetic temperature calculated to be not more than 25°C; and that allows for excursions between 15°C and 30°C (59° and 86°F) that are experienced in pharmacies, hospitals, and warehouses. Provided the mean kinetic temperature remains in the allowed range, transient spikes up to 40°C are permitted as long as they do not exceed 24 hours ... The mean kinetic temperature is a calculated value that may be used as an isothermal storage temperature that simulates the non isothermal effects of storage temperature variations.”
Reference: United States Pharmacopeia, General Notices. United States Pharmacopeial Convention, Inc. 12601 Twinbrook Parkway, Rockville, MD.
1 Van der Meer, P.F., & de Korte, D. “Increase of blood donation speed by optimizing the needle-totubing connection: an application of donation software.” Vox Sanguinis 2009, 97: 21-25
Sorvall is a trademark of Thermo Fisher Scientific LLC.
© 2019 Fresenius Kabi AG. All rights reserved.
-
PACKAGE/LABEL DISPLAY PANEL
Code 4R3468 15 Units
Fresenius Kabi
Fenwal Blood-Pack Units Double
Anticoagulant Citrate Phosphate Dextrose Solution, USP (CPD); Satellite Container with Adsol Red Cell Preservation Solution
For the Collection and Processing of 500 mL Blood
Sample Diversion System, 16 ga. Ultra Thin Wall Fenwal HighFlo Needle
Rx only
Each unit consists of a primary container with 70 mL of CPD solution containing 1.84 g Sodium Citrate (dihydrate) USP, 1.78 g Dextrose (monohydrate) USP, 209 mg Citric Acid (anhydrous) USP, 155 mg Monobasic Sodium Phosphate (monohydrate) USP, pH may have been adjusted with sodium hydroxide; one satellite container with 110 mL of Adsol Red Cell Preservation Solution containing 2.42 g Dextrose (monohydrate) USP, 990 mg Sodium Chloride USP, 825 mg Mannitol USP, 30 mg Adenine USP; one empty 400 mL Transfer-Pack container.
Sterile, non-pyrogenic fluid path. See instructions for use.
Single use only.
Store at Controlled Room Temperature (refer to direction insert).
- •
- Open pouch by tearing across at notch.
- •
- Direct handling of product surfaces prior to extended storage in the foil pouch, may result in mold growth.
- •
- Unused units in open foil pouch may be kept up to 60 days by folding and securing open end of foil pouch to prevent possible loss of moisture, provided:
- I)
- Units are not removed from foil pouch, or
- II)
- Unused units removed from foil pouch are returned to the foil pouch within 12 hours. Units may be removed from the pouch and returned only once.
- •
- Units removed from the foil pouch (that are not returned to the pouch within 12 hours) must be used within 4 days (96 hours). Units out of the foil pouch for longer than 96 hours must be discarded.
- Manufacturer
- Fresenius Kabi AG
- 61346 Bad Homburg / Germany
www.fresenius-kabi.com
Made in US
47-28-13-462 REV: A
-
INGREDIENTS AND APPEARANCE
ADSOL RED CELL PRESERVATION SOLUTION SYSTEM IN PLASTIC CONTAINER (PL 146 PLASTIC)
anticoagulant citrate phosphate dextrose (cpd) solution and adsol preservation solution kitProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0942-6461 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0942-6461-02 1 in 1 KIT; Type 0: Not a Combination Product Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BAG 70 mL Part 2 1 BAG 110 mL Part 1 of 2 CPD
citrate phosphate dextrose solutionProduct Information Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Trisodium Citrate Dihydrate (UNII: B22547B95K) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 1.84 g in 70 mL Dextrose Monohydrate (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) Dextrose Monohydrate 1.78 g in 70 mL Anhydrous Citric Acid (UNII: XF417D3PSL) (Anhydrous Citric Acid - UNII:XF417D3PSL) Anhydrous Citric Acid 209 mg in 70 mL Sodium Phosphate, Monobasic, Monohydrate (UNII: 593YOG76RN) (PHOSPHATE ION - UNII:NK08V8K8HR) Sodium Phosphate, Monobasic, Monohydrate 155 mg in 70 mL Inactive Ingredients Ingredient Name Strength Sodium Hydroxide (UNII: 55X04QC32I) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 70 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN811104 03/01/2007 Part 2 of 2 ADSOL RED CELL PRESERVATION SOLUTION SYSTEM
adsol red cell preservation solution solutionProduct Information Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dextrose Monohydrate (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) Dextrose Monohydrate 2.42 g in 110 mL Sodium Chloride (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) Sodium Chloride 990 mg in 110 mL Mannitol (UNII: 3OWL53L36A) (Mannitol - UNII:3OWL53L36A) Mannitol 825 mg in 110 mL Adenine (UNII: JAC85A2161) (Adenine - UNII:JAC85A2161) Adenine 30 mg in 110 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 110 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN811104 03/01/2007 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA BN811104 03/01/2007 Labeler - Fenwal, Inc. (794519020) Establishment Name Address ID/FEI Business Operations Fenwal International, Inc. 091164590 MANUFACTURE