Label: ILARIS- canakinumab injection, solution
- NDC Code(s): 0078-0734-61
- Packager: Novartis Pharmaceuticals Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated June 18, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ILARIS safely and effectively. See full prescribing information for ILARIS.
ILARIS® (canakinumab) injection, for subcutaneous use
Initial U.S. Approval: 2009RECENT MAJOR CHANGES
Warnings and Precautions, Hypersensitivity Reactions (5.3) 11/2024
INDICATIONS AND USAGE
ILARIS is an interleukin-1β blocker indicated for the treatment of:
• Periodic Fever Syndromes (1.1):
- Cryopyrin-Associated Periodic Syndromes (CAPS), in adults and children 4 years of age and older, including:
◾ Familial Cold Auto-inflammatory Syndrome (FCAS)
◾ Muckle-Wells Syndrome (MWS)
- Tumor Necrosis Factor Receptor Associated Periodic Syndrome (TRAPS) in adult and pediatric patients
- Hyperimmunoglobulin D Syndrome (HIDS)/Mevalonate Kinase Deficiency (MKD) in adult and pediatric patients
- Familial Mediterranean Fever (FMF) in adult and pediatric patients
• Active Still’s Disease, including Adult-Onset Still’s Disease (AOSD) and Systemic Juvenile Idiopathic Arthritis (SJIA) in patients 2 years of age and older (1.2)
• Gout flares in adults in whom non-steroidal anti-inflammatory drugs (NSAIDs) and colchicine are contraindicated, are not tolerated, or do not provide an adequate response, and in whom repeated courses of corticosteroids are not appropriate (1.3)
DOSAGE AND ADMINISTRATION
• CAPS: Recommended weight-based dosage is:
- For patients > 40 kg: 150 mg subcutaneously, every 8 weeks
- For patients ≥ 15 kg and < 40 kg: 2 mg/kg subcutaneously, every 8 weeks. For pediatric patients 15 kg to 40 kg with an inadequate response, the dose can be increased to 3 mg/kg. (2.2)
• TRAPS, HIDS/MKD, and FMF: Recommended weight-based dosage is:
- For patients > 40 kg: Starting dosage is 150 mg subcutaneously every 4 weeks. The dosage can be increased to 300 mg every 4 weeks if the clinical response is not adequate. (2.3)
- For patients ≤ 40 kg: Starting dosage is 2 mg/kg subcutaneously every 4 weeks. The dosage can be increased to 4 mg/kg every 4 weeks if the clinical response is not adequate. (2.3)
- Still’s disease (AOSD and SJIA): Recommended weight-based dosage for patients ≥ 7.5 kg is 4 mg/kg (maximum dose of 300 mg), subcutaneously, every 4 weeks. (2.4)
- Gout Flares: Recommended dosage is 150 mg subcutaneously. In patients who require re-treatment, there should be an interval of at least 12 weeks before a new dose of ILARIS may be administered. (2.5)
DOSAGE FORMS AND STRENGTHS
- Injection: 150 mg/mL solution in single-dose vials. (3)
CONTRAINDICATIONS
Confirmed hypersensitivity to canakinumab or to any of the excipients. (4)
WARNINGS AND PRECAUTIONS
- Serious Infections: ILARIS has been associated with an increased incidence of serious infections. Exercise caution when administering ILARIS to patients with infections, a history of recurring infections or underlying conditions which may predispose them to infections. Discontinue ILARIS if a patient develops a serious infection. Avoid administering ILARIS to patients during an active infection requiring medical intervention. (5.1)
- Hypersensitivity Reactions and Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) can occur; discontinue ILARIS, treat promptly, and monitor until reaction resolves. (5.3)
- Immunizations: Avoid administration of live vaccines concurrently with ILARIS. Update all recommended vaccinations prior to initiation of therapy with ILARIS. (5.4)
ADVERSE REACTIONS
- CAPS: The most common adverse reactions (>10%) are nasopharyngitis, diarrhea, influenza, rhinitis, nausea, headache, bronchitis, gastroenteritis, pharyngitis, weight increased, musculoskeletal pain, and vertigo. (6)
- TRAPS, HIDS/MKD, and FMF: The most common adverse reactions (≥10%) are injection-site reactions and nasopharyngitis. (6)
- Still’s Disease: The most common adverse drug reactions (>10%) are infections (nasopharyngitis and upper respiratory tract infections), abdominal pain, and injection-site reactions. (6)
- Gout Flares: The most common adverse reactions (>2%) reported by are nasopharyngitis, upper respiratory tract infections, urinary tract infections, hypertriglyceridemia, and back pain. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Periodic Fever Syndromes
1.2 Still’s Disease (Adult-Onset Still’s Disease [AOSD] and Systemic Juvenile Idiopathic Arthritis [SJIA])
1.3 Gout Flares
2 DOSAGE AND ADMINISTRATION
2.1 General Dosing Information
2.2 Recommended Dosage for Cryopyrin-Associated Periodic Syndromes (CAPS)
2.3 Recommended Dosage for Tumor Necrosis Factor Receptor Associated Periodic Syndrome (TRAPS), Hyperimmunoglobulin D Syndrome/Mevalonate Kinase Deficiency (HIDS/MKD), and Familial Mediterranean Fever (FMF)
2.4 Recommended Dosage for Still’s Disease, Including Adult-Onset Still’s Disease (AOSD) and Systemic Juvenile Idiopathic Arthritis (SJIA)
2.5 Recommended Dosage for Gout Flares
2.6 Administration Instructions for ILARIS Injection
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Infections
5.2 Immunosuppression
5.3 Hypersensitivity Reactions
5.4 Immunizations
5.5 Macrophage Activation Syndrome
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
6.3 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 TNF-Blocker and IL-1 Blocking Agent
7.2 Immunization
7.3 Cytochrome P450 Substrates
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Treatment of CAPS
14.2 Treatment of Periodic Fever Syndromes: TRAPS, HIDS/MKD, and FMF
14.3 Treatment of Still’s Disease: AOSD and SJIA
14.4 Treatment of Gout Flares
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1
INDICATIONS AND USAGE
1.1 Periodic Fever Syndromes
ILARIS® (canakinumab) is an interleukin-1β (IL-1β) blocker indicated for the treatment of the following autoinflammatory Periodic Fever Syndromes:
Cryopyrin-Associated Periodic Syndromes (CAPS)
ILARIS is indicated for the treatment of Cryopyrin-Associated Periodic Syndromes (CAPS), in adults and pediatric patients 4 years of age and older, including:
- Familial Cold Autoinflammatory Syndrome (FCAS)
- Muckle-Wells Syndrome (MWS)
Tumor Necrosis Factor Receptor (TNF) Associated Periodic Syndrome (TRAPS)
ILARIS is indicated for the treatment of Tumor Necrosis Factor (TNF) Receptor Associated Periodic Syndrome (TRAPS) in adult and pediatric patients.
Hyperimmunoglobulin D Syndrome (HIDS)/Mevalonate Kinase Deficiency (MKD)
ILARIS is indicated for the treatment of Hyperimmunoglobulin D (Hyper-IgD) Syndrome (HIDS)/Mevalonate Kinase Deficiency (MKD) in adult and pediatric patients.
Familial Mediterranean Fever (FMF)
ILARIS is indicated for the treatment of Familial Mediterranean Fever (FMF) in adult and pediatric patients.
1.2 Still’s Disease (Adult-Onset Still’s Disease [AOSD] and Systemic Juvenile Idiopathic Arthritis [SJIA])
ILARIS is indicated for the treatment of active Still’s Disease, including Adult-Onset Still’s Disease (AOSD) and Systemic Juvenile Idiopathic Arthritis (SJIA) in patients 2 years of age and older.
1.3 Gout Flares
ILARIS is indicated for the symptomatic treatment of adult patients with gout flares in whom non-steroidal anti-inflammatory drugs (NSAIDs) and colchicine are contraindicated, are not tolerated, or do not provide an adequate response, and in whom repeated courses of corticosteroids are not appropriate.
-
2
DOSAGE AND ADMINISTRATION
2.2 Recommended Dosage for Cryopyrin-Associated Periodic Syndromes (CAPS)
The recommended weight-based dosage of ILARIS is:
- For patients with CAPS > 40 kg: 150 mg subcutaneously, every 8 weeks
- For patients with CAPS ≥ 15 kg and ≤ 40 kg: 2 mg/kg subcutaneously, every 8 weeks.
- For pediatric patients with CAPS 15 kg to 40 kg with an inadequate response, the dosage can be increased to 3 mg/kg subcutaneously, every 8 weeks.
2.3 Recommended Dosage for Tumor Necrosis Factor Receptor Associated Periodic Syndrome (TRAPS), Hyperimmunoglobulin D Syndrome/Mevalonate Kinase Deficiency (HIDS/MKD), and Familial Mediterranean Fever (FMF)
The recommended weight-based dosage of ILARIS for patients with TRAPS, HIDS/MKD, and FMF is:
- For patients > 40 kg: 150 mg subcutaneously, every 4 weeks. The dosage can be increased to 300 mg every 4 weeks if the clinical response is not adequate.
- For patients ≤ 40 kg: 2 mg/kg administered subcutaneously, every 4 weeks. The dosage can be increased to 4 mg/kg every 4 weeks if the clinical response is not adequate.
2.4 Recommended Dosage for Still’s Disease, Including Adult-Onset Still’s Disease (AOSD) and Systemic Juvenile Idiopathic Arthritis (SJIA)
The recommended weight-based dosage of ILARIS for patients with Still’s Disease (AOSD and SJIA) weighing ≥ 7.5 kg is 4 mg/kg (maximum dose of 300 mg) administered subcutaneously every 4 weeks.
2.5 Recommended Dosage for Gout Flares
The recommended dose of ILARIS for adult patients with a gout flare is 150 mg administered subcutaneously. In patients who require re-treatment, there should be an interval of at least 12 weeks before a new dose of ILARIS may be administered.
2.6 Administration Instructions for ILARIS Injection
STEP 1: ILARIS injection has a concentration of 150 mg/mL. Do not shake. The solution should be essentially free from particulates, clear to opalescent, colorless to slightly brownish-yellow tint. If the solution has a distinctly brown discoloration, is highly opalescent or contains visible particles, do not use.
STEP 2: Using a sterile 1-mL syringe and 18-gauge x 2” needle, carefully withdraw the required volume depending on the dose to be administered and subcutaneously inject using a 27-gauge x 0.5” needle.
Avoid injection into scar tissue as this may result in insufficient exposure to ILARIS.
Discard unused product or waste material in accordance with the local requirements.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5
WARNINGS AND PRECAUTIONS
5.1 Serious Infections
ILARIS has been associated with an increased risk of serious infections. Exercise caution when administering ILARIS to patients with infections, a history of recurring infections or underlying conditions which may predispose them to infections. Avoid administering ILARIS to patients during an active infection requiring medical intervention. Discontinue ILARIS if a patient develops a serious infection.
Infections, predominantly of the upper respiratory tract, in some instances serious, have been reported with ILARIS. Generally, the observed infections responded to standard therapy. Isolated cases of unusual or opportunistic infections (e.g., aspergillosis, atypical mycobacterial infections, cytomegalovirus, herpes zoster) were reported during ILARIS treatment. A causal relationship of ILARIS to these events cannot be excluded. In clinical trials, ILARIS has not been administered concomitantly with tumor necrosis factor (TNF) inhibitors. An increased incidence of serious infections has been associated with administration of another IL-1 blocker in combination with TNF inhibitors. Coadministration of ILARIS with TNF inhibitors is not recommended because this may increase the risk of serious infections [see Drug Interactions (7.1)].
Drugs that affect the immune system by blocking TNF have been associated with an increased risk of new tuberculosis and reactivation of latent tuberculosis (TB). It is possible that use of IL-1 inhibitors, such as ILARIS, increases the risk of reactivation of tuberculosis or of opportunistic infections.
Prior to initiating immunomodulatory therapies, including ILARIS, evaluate patients for active and latent tuberculosis infection. Appropriate screening tests should be performed in all patients. ILARIS has not been studied in patients with a positive tuberculosis screen, and the safety of ILARIS in individuals with latent tuberculosis infection is unknown. Treat patients testing positive in tuberculosis screening according to standard medical practice prior to therapy with ILARIS. Instruct patients to seek medical advice if signs, symptoms, or high-risk exposure suggestive of tuberculosis (e.g., persistent cough, weight loss, subfebrile temperature) appear during or after ILARIS therapy.
Healthcare providers should follow current CDC guidelines both to evaluate for and to treat possible latent tuberculosis infections before initiating therapy with ILARIS.
5.2 Immunosuppression
The impact of treatment with anti-interleukin-1 (IL-1) therapy on the development of malignancies is not known. However, treatment with immunosuppressants, including ILARIS, may result in an increase in the risk of malignancies.
5.3 Hypersensitivity Reactions
Hypersensitivity reactions have been reported with ILARIS. During clinical trials, no anaphylactic reactions attributable to treatment with canakinumab have been reported. It should be recognized that symptoms of the underlying disease being treated may be similar to symptoms of hypersensitivity [see Adverse Reactions (6.1)]. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), characterized by serious skin eruptions, has been reported in patients with autoinflammatory conditions treated with ILARIS. If a severe hypersensitivity reaction occurs, immediately discontinue ILARIS; treat promptly and monitor until signs and symptoms resolve.
5.4 Immunizations
Avoid administration of live vaccines concurrently with ILARIS [see Drug Interactions (7.2)]. Since no data are available on either the efficacy or on the risks of secondary transmission of infection by live vaccines in patients receiving ILARIS, avoid administering live vaccines concurrently with ILARIS. In addition, because ILARIS may interfere with normal immune response to new antigens, vaccinations may not be effective in patients receiving ILARIS. Limited data are available on the response to vaccinations with inactivated (killed) antigens in patients receiving ILARIS [see Drug Interactions (7.2)].
Because IL-1 blockade may interfere with immune response to infections, it is recommended that prior to initiation of therapy with ILARIS, adult and pediatric patients receive all recommended vaccinations, as appropriate and if feasible, including pneumococcal vaccine and inactivated influenza vaccine. See current recommended immunization schedules at the website of the Centers for Disease Control, http://www.cdc.gov/vaccines/schedules/index.html.
5.5 Macrophage Activation Syndrome
Macrophage activation syndrome (MAS) is a known, life-threatening disorder that may develop in patients with rheumatic conditions, in particular Still’s disease, and should be aggressively treated. Physicians should be attentive to symptoms of infection or worsening of Still’s disease, as these are known triggers for MAS. Eleven cases of MAS were observed in 201 SJIA patients treated with canakinumab in clinical trials. Based on the clinical trial experience, ILARIS does not appear to increase the incidence of MAS in Still’s disease patients, but no definitive conclusion can be made.
-
6
ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Serious Infections [see Warnings and Precautions (5.1)]
- Immunosuppression [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Macrophage Activation Syndrome [see Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions from Clinical Trials for Treatment of Periodic Fever Syndromes: CAPS, TRAPS, HIDS/MKD, and FMF
Treatment of CAPS
The data described herein reflect exposure to ILARIS in 104 adult and pediatric CAPS patients, including 20 FCAS, 72 MWS, 10 MWS/NOMID (Neonatal Onset Multisystem Inflammatory Disorder) overlap, 1 non-FCAS non-MWS, and 1 misdiagnosed in placebo-controlled (35 patients) and uncontrolled trials. Sixty-two patients were exposed to ILARIS for at least 6 months, 56 for at least 1 year, and 4 for at least 3 years. A total of 9 serious adverse reactions were reported for CAPS patients. Among these were vertigo (2 patients), infections (3 patients), including intra-abdominal abscess following appendectomy (1 patient). The most commonly reported adverse reactions associated with ILARIS treatment in greater than 10% of the CAPS patients were nasopharyngitis, diarrhea, influenza, rhinitis, nausea, headache, bronchitis, gastroenteritis, pharyngitis, weight increased, musculoskeletal pain, and vertigo. One patient discontinued treatment due to potential infection.
CAPS Study 1 investigated the safety of ILARIS in an 8-week, open-label period (Part 1), followed by a 24-week, randomized withdrawal period (Part 2), followed by a 16-week, open-label period (Part 3). All patients were treated with ILARIS 150 mg subcutaneously or 2 mg/kg if body weight was greater than or equal to 15 kg and less than or equal to 40 kg (see Table 1).
Since all CAPS patients received ILARIS in Part 1, there are no controlled data on adverse events (AEs). Data in Table 1 are for all AEs for all CAPS patients receiving canakinumab. In CAPS Study 1, no pattern was observed for any type or frequency of adverse events throughout the 3 study periods.
Table 1: Adverse Reactions in ≥ 10% of Patients in Parts 1 to 3 of the Phase 3 Trial for Patients with CAPS
Adverse reactionsILARIS
N = 35
n (%)n (%) of patients with adverse reactions 35 (100) Nasopharyngitis 12 (34) Diarrhea 7 (20) Influenza 6 (17) Rhinitis 6 (17) Nausea 5 (14) Headache 5 (14) Bronchitis 4 (11) Gastroenteritis 4 (11) Pharyngitis 4 (11) Weight increased 4 (11) Musculoskeletal pain 4 (11) Vertigo 4 (11) Vertigo
Vertigo has been reported in 9% to 14% of patients in CAPS studies, exclusively in MWS patients, and reported as a serious adverse event in 2 cases. All events resolved with continued treatment with ILARIS.
Injection-Site Reactions
In CAPS Study 1, subcutaneous injection-site reactions were observed in 9% of patients in Part 1 with mild tolerability reactions; in Part 2, one patient each (7%) had a mild or a moderate tolerability reaction and, in Part 3, one patient had a mild local tolerability reaction. No severe injection-site reactions were reported, and none led to discontinuation of treatment.
Treatment of TRAPS, HIDS/MKD, and FMF
A Phase 3 trial (TRAPS, HIDS/MKD, and FMF Study 1) investigated the safety of ILARIS in 3 cohorts (TRAPS, HIDS/MKD, and FMF) as follows: a 12-week screening period (Part 1), followed by a 16-week, randomized, double-blind, placebo-controlled parallel-arm treatment period (Part 2), followed by a 24-week randomized withdrawal period (Part 3), followed by a 72-week, open-label treatment period (Part 4). All patients randomized to treatment with ILARIS in Part 2 received 150 mg subcutaneously every 4 weeks if body weight was greater than 40 kg (or 2 mg/kg every 4 weeks if body weight was less than or equal to 40 kg).
In Part 2 of the TRAPS, HIDS/MKD, and FMF Study 1, initially 90 patients were randomized to ILARIS treatment, and 91 patients were randomized to placebo. Of patients randomized to ILARIS, 55.6% remained on the initial dose through Week 16 with 6.7% receiving an additional ILARIS dose between Day 7 and Day 15. Of the patients randomized to placebo, 9.9% remained on placebo through Week 16 with 28.6% switching to active treatment with ILARIS by Day 15.
Overall, there were 43 TRAPS, 68 HIDS/MKD, and 58 FMF patients in the safety set with a cumulative canakinumab exposure of 47.61 patient-years. The cumulative exposure in the placebo group was 8.03 patient-years.
In Part 2 of the TRAPS, HIDS/MKD, and FMF Study 1, a total of 22 TRAPS patients aged 3 to 76 years of age, 37 HIDS/MKD patients aged 2 to 43 years of age, and 31 FMF patients aged 2 to 60 years of age were initially randomized to treatment with ILARIS 150 mg every four weeks in the placebo-controlled period of the clinical trial. In addition, 4 non-randomized patients (2 FMF patients of age 20 and 29 years with non-exon 10 mutations and 2 HIDS/MKD patients both of 1 year of age) received open-label treatment in Part 2.
The most commonly reported adverse reactions (greater than or equal to 10%) associated with ILARIS treatment in TRAPS, HIDS/MKD, and FMF patients were injection-site reactions and nasopharyngitis. The reported adverse reactions (greater than or equal to 3%) associated with ILARIS treatment in TRAPS, HIDS/MKD, and FMF patients were injection-site reactions (10.1%), and infections, including nasopharyngitis (10.7%), upper respiratory tract infection (7.1%), rhinitis (5.3%), gastroenteritis (3.0%), and pharyngitis (3.0%). Serious infections (e.g., conjunctivitis, pneumonia, pharyngitis, pharyngotonsillitis) were observed in approximately 2.4% (0.03 per 100 patient-days) of patients receiving ILARIS in Part 2 of the TRAPS, HIDS/MKD, and FMF Study 1.
In the ILARIS treatment group, 1 TRAPS patient discontinued treatment due to adverse events, 2 HIDS/MKD patients discontinued treatment due to adverse events, and no FMF patients discontinued treatment due to an adverse event.
Injection-Site Reactions
In the TRAPS, HIDS/MKD, and FMF Study 1, subcutaneous injection-site reactions were observed in 10.1% of patients in Part 2 who had a mild or a moderate tolerability reaction. No severe injection-site reactions were reported and none led to discontinuation of treatment.
Adverse Reactions from Clinical Trials for Treatment of Still’s Disease: SJIA and AOSD
The safety of ILARIS compared to placebo in SJIA patients was investigated in two Phase 3 studies [see Clinical Studies (14.2)]. Patients in SJIA Study 1 received a single dose of ILARIS 4 mg/kg (n = 43) or placebo (n = 41) via subcutaneous injection and were assessed at Day 15 for the efficacy endpoints and had a safety analysis up to Day 29. SJIA Study 2 was a two-part study with an open-label, single-arm active treatment period (Part I) followed by a randomized, double-blind, placebo-controlled, event-driven withdrawal design (Part II). Overall, 177 patients were enrolled into the study and received ILARIS 4 mg/kg (up to 300 mg maximum) in Part I, and 100 patients received ILARIS 4 mg/kg (up to 300 mg maximum) every 4 weeks or placebo in Part II. Adverse drug reactions listed in Table 2 showed higher rates than placebo from both trials. The adverse drug reactions associated with ILARIS treatment in greater than 10% of SJIA patients were infections, abdominal pain, and injection-site reactions. Serious infections (e.g., pneumonia, varicella, gastroenteritis, measles, sepsis, otitis media, sinusitis, adenovirus, lymph node abscess, pharyngitis) were observed in approximately 4% to 5% (0.02 to 0.17 per 100 patient-days) of patients receiving ILARIS in both studies.
Table 2: Tabulated Summary of Adverse Drug Reactions From Pivotal SJIA Clinical Trials n = number of patients.
^IR = Exposure adjusted incidence rate per 100 patient-days.
*No injection-site reaction led to study discontinuation.SJIA Study 2 SJIA Study 1 Part I Part II ILARIS
N = 177
n (%)
(IR)^ILARIS
N = 50
n (%)
(IR)Placebo
N = 50
n (%)
(IR)ILARIS
N = 43
n (%)
(IR)Placebo
N = 41
n (%)
(IR)Infections and infestations All infections (e.g., nasopharyngitis, [viral] upper respiratory tract infection, pneumonia, rhinitis, pharyngitis, tonsillitis, sinusitis, urinary tract infection, gastroenteritis, viral infection) 97 (54.8%)
(0.91)27 (54%)
(0.59)19 (38%)
(0.63)13 (30.2%)
(1.26)5 (12.2%)
(1.37)Gastrointestinal disorders Abdominal pain (upper) 25 (14.1%)
(0.16)8 (16%)
(0.15)6 (12%)
(0.08)3 (7%)
(0.25)1 (2.4%)
(0.23)Skin and subcutaneous tissue disorders Injection-site reaction* mild 19 (10.7%) 6 (12.0%) 2 (4.0%) 0 3 (7.3%) moderate 2 (1.1%) 1 (2.0%) 0 0 0 The safety profile of ILARIS in AOSD patients in a randomized, double-blind, placebo-controlled study (GDE01T) in 36 adult patients (aged 22 to 70 years) was similar to what was observed in SJIA patients.
Adverse Reactions from Clinical Trials for Treatment of Gout Flares
The safety of ILARIS compared to triamcinolone acetonide in patients with gout flares was assessed in four 12-week randomized, double-blind, active-controlled Phase 3 studies [see Clinical Studies (14.4) for details of the studies supporting efficacy] and in two 12-week double-blind active-controlled extension studies. In the ILARIS treatment groups 512 patients were treated up to 12 weeks and 165 of these patients up to 24 weeks. In the triamcinolone acetonide groups, 381 patients were treated up to 12 weeks and 152 of these patients up to 24 weeks. Patients received a single dose of ILARIS 150 mg (n = 467) via subcutaneous injection or triamcinolone acetonide 40 mg (n = 279) via intramuscular injection. Upon a new flare, 85 and 152 patients received at least one additional dose of ILARIS and triamcinolone acetonide, respectively.
The most commonly reported adverse drug reactions were infections and infestations (see Table 3). The most common infections reported in more than 2% of patients in the ILARIS treatment groups were nasopharyngitis, upper respiratory tract infections, and urinary tract infections. The trends observed in all infections are aligned with the overall known safety profile of canakinumab. Serious adverse events were reported in 1.4% of the ILARIS-treated patients, all of which were single events. No serious adverse events were reported in the triamcinolone acetonide-treated group.
Of the ILARIS-treated patients, 17% were 65 years of age and older, including 3% who were 75 years of age and older. No new safety findings were observed between these patients compared to patients under 65 years of age [see Use in Specific Populations (8.5).
Table 3: Tabulated Summary of Adverse Drug Reactions From Pivotal Gout Flare Clinical Trials Abbreviation: SOC, system organ class.
*N = Number of patients at study entry.
IR-w = Study size weighted incidence rate (i.e., number of patients with an event per 100 patient-years).System Organ Class
Adverse reactionILARIS 150 mg
*N = 552
n (%)
(IR-w)Triamcinolone acetonide 40 mg
*N = 431
n (%)
(IR-w)Infections and infestations All infections (e.g., nasopharyngitis, upper respiratory tract infection, urinary tract infections) 90 (16.3%)
(59.0)40 (9.3%)
(32.1)Investigations Blood triglycerides increased 7 (1.3%)
(3.8)2 (0.5%)
(1.3)Platelet count decreased 4 (0.7%)
(2.5)1 (0.2%)
(1.0)Metabolism and nutrition disorders Hypertriglyceridemia 15 (2.7%)
(9.5)4 (0.9%)
(3)Musculoskeletal and connective tissue disorders Back pain 17 (3.1%)
(10.9)7 (1.6%)
(6.2)Nervous system disorders Dizziness 9 (1.6%)
(5.8)2 (0.5%)
(1.7)Specific Adverse Reactions from Clinical Trials
Hypersensitivity
During clinical trials, no anaphylactic reactions attributable to treatment with canakinumab have been reported. In CAPS trials one patient discontinued and in TRAPS, HIDS/MKD, FMF, Still’s disease, and gout trials no patients discontinued due to hypersensitivity reactions. ILARIS should not be administered to any patients with known clinical hypersensitivity to ILARIS [see Contraindications (4) and Warnings and Precautions (5.3)].
Laboratory Abnormalities
- Hematology
TRAPS, HIDS/MKD, and FMF
Overall, in the TRAPS, HIDS/MKD, and FMF Study 1, neutrophil count decreased (greater than or equal to Grade 2) was reported in 6.5% of patients and platelet count decreased (greater than or equal to Grade 2) was reported in 0.6% of patients.
SJIA
During clinical trials with ILARIS, mean values decreased for white blood cells, neutrophils and platelets.
In the randomized, placebo-controlled portion of SJIA Study 2, decreased white blood cell counts (WBC) less than or equal to 0.8 times lower limit of normal (LLN) were reported in 5 patients (10.4%) in the ILARIS group compared to 2 patients (4.0%) in the placebo group. Transient decreases in absolute neutrophil count (ANC) to less than 1 x 109/L were reported in 3 patients (6.0%) in the ILARIS group compared to 1 patient (2.0%) in the placebo group. One case of ANC less than 0.5x109/L was observed in the ILARIS group and none in the placebo group.
Mild (less than LLN and greater than 75 x 109/L) and transient decreases in platelet counts were observed in 3 (6.3%) ILARIS-treated patients versus 1 (2.0%) placebo-treated patient.
Gout Flares
In the pooled analysis of patients with gout flares from four 12-week randomized, double-blind, active-controlled Phase 3 studies and two 12-week active-controlled extension studies, transient cytopenias were observed. Leukopenia (WBC ≤ 0.8 x LLN) was reported in 6.4% of ILARIS-treated patients compared to 1.4% of triamcinolone acetonide-treated patients. Neutropenia (ANC < 0.9 x LLN) was reported in 15.9% of patients treated with ILARIS compared to 2.1% treated with triamcinolone acetonide. Thrombocytopenia (platelet counts < LLN) was observed in 16.3% of patients treated with ILARIS versus 12.5% of patients treated with triamcinolone acetonide.
- Uric Acid
Gout Flares
The proportion of patients with laboratory abnormalities (from normal at baseline to >ULN or from ≤9.9 mg/dl at baseline to > 9.9 mg/dl) and/or adverse reactions of increased uric acid levels were numerically higher in the ILARIS group (43.8% for ILARIS vs. 40.1% for (triamcinolone acetonide).
- Hepatic Transaminases
Elevations of transaminases (ALT/AST) have been observed in patients treated with ILARIS.
SJIA
In the randomized, placebo-controlled portion of SJIA Study 2, high ALT and/or AST ≥ 3 times upper limit of normal (ULN) were reported in 2 (4.1%) ILARIS-treated patients and 1 (2.0%) placebo patient. All patients had normal values at the next visit.
Gout Flares
In the randomized double-blind studies up to 24 weeks high ALT and AST ≥ 3 times upper limit of normal (ULN) were reported in 1.6% and 0.5% of ILARIS-treated patients respectively, and 2.6% and 1.9% of the triamcinolone acetonide-treated patients, respectively.
- Bilirubin
SJIA
Asymptomatic and mild elevations of serum bilirubin have been observed in patients treated with ILARIS without concomitant elevations of transaminases.
- Hypertriglyceridemia
Gout Flares
The proportion of patients with hypertriglyceridemia events in the randomized double-blind studies up to 24 weeks was higher in the ILARIS group compared to the triamcinolone acetonide-treated group (5.6% vs. 1.9%). The majority of abnormal values were noted at a single visit.
6.2 Immunogenicity
A biosensor binding assay or a bridging immunoassay was used to detect antibodies directed against canakinumab in patients who received ILARIS. Following treatment with ILARIS, antibodies against ILARIS were observed in approximately 1.4%, 1.2%, and 3.5% of the patients with CAPS, SJIA, and gout flares, respectively. Neutralizing antibodies were detected in < 1% of patients with gout flares. No apparent correlation of antibody development to clinical response or adverse events was observed. The CAPS clinical studies employed the biosensor binding assay, most of the SJIA clinical studies employed the bridging assay, and the gout clinical studies used initially the biosensor assay and for later studies or extensions the bridging assay. The data obtained in an assay are highly dependent on several factors, including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medications, underlying disease, and the number of patients tested. For these reasons, comparison of the incidence of antibodies to canakinumab between the CAPS, SJIA, and gout flare clinical studies or with the incidence of antibodies to other products may be misleading.
No TRAPS, HIDS/MKD, FMF, SJIA, or AOSD patients treated with ILARIS doses of 150 mg and 300 mg over 16 weeks of treatment tested positive for anti-canakinumab antibodies.
6.3 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of ILARIS. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and subcutaneous tissue disorders:
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) [see Warnings and Precautions (5.3)]
-
7
DRUG INTERACTIONS
Interactions between ILARIS and other medicinal products have not been investigated in formal studies.
7.1 TNF-Blocker and IL-1 Blocking Agent
An increased incidence of serious infections and an increased risk of neutropenia have been associated with administration of another IL-1 blocker in combination with TNF inhibitors in another patient population. Use of ILARIS with TNF inhibitors may also result in similar toxicities and is not recommended because this may increase the risk of serious infections [see Warnings and Precautions (5.1)].
The concomitant administration of ILARIS with other drugs that block IL-1 has not been studied. Based upon the potential for pharmacological interactions between ILARIS and a recombinant IL-1ra, concomitant administration of ILARIS and other agents that block IL-1 or its receptors is not recommended.
7.2 Immunization
No data are available on either the effects of live vaccination or the secondary transmission of infection by live vaccines in patients receiving ILARIS. Therefore, avoid administration of live vaccines concurrently with ILARIS. It is recommended that, if possible, pediatric and adult patients complete all immunizations in accordance with current immunization guidelines prior to initiating ILARIS therapy [see Warnings and Precautions (5.4)].
7.3 Cytochrome P450 Substrates
The formation of CYP450 enzymes is suppressed by increased levels of cytokines (e.g., IL-1) during chronic inflammation. Thus, it is expected that for a molecule that binds to IL-1, such as canakinumab, the formation of CYP450 enzymes could be normalized. This is clinically relevant for CYP450 substrates with a narrow therapeutic index, where the dose is individually adjusted (e.g., warfarin). Upon initiation of canakinumab, in patients being treated with these types of medicinal products, therapeutic monitoring of the effect or drug concentration should be performed, and the individual dose of the medicinal product may need to be adjusted as needed.
-
8
USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available human data from postmarketing experience and published case reports on ILARIS use in pregnant women are insufficient to identify a drug-associated risk of major birth defects, miscarriage, and adverse maternal or fetal outcomes. Canakinumab, like other monoclonal antibodies, is actively transported across the placenta mainly during the third trimester of pregnancy and may cause immunosuppression in the in utero exposed infant (see Clinical Considerations).
In an animal embryo-fetal development study with marmoset monkeys, there was no evidence of embryotoxicity or fetal malformations with subcutaneous administration of canakinumab during the period of organogenesis and later in gestation at doses that produced exposures approximately 11 times the exposure at the maximum recommended human dose (MRHD) and greater. Delays in fetal skeletal development were observed in marmoset monkeys following prenatal exposure to ILARIS at concentrations approximately 11 times the MRHD and greater. Similar delays in fetal skeletal development were observed in mice administered a murine analog of ILARIS during the period of organogenesis. Delays in skeletal ossification are changes from the expected ossification state in an otherwise normal structure/bone: these findings are generally reversible or transitory and not detrimental to postnatal survival (see Animal Data).
The estimated background risk of major birth defects and miscarriage for the indicated population(s) are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Monoclonal antibodies are increasingly transported across the placenta as pregnancy progresses, with the largest amount transferred during the third trimester. Because IL-1 blockade may interfere with immune response to infections, risks and benefits should be considered prior to administering live vaccines to infants who were exposed to ILARIS in utero for at least 4 to 12 months following the mother’s last dose of ILARIS. The ideal time to avoid live vaccines in infants exposed to ILARIS in utero is unknown, as there are insufficient data regarding infant serum levels of canakinumab at birth and the duration of persistence of canakinumab in infant serum after birth is also unknown.
Data
Animal Data
In an embryo-fetal development study, pregnant marmoset monkeys received canakinumab from gestation days 25 to 109 at doses that produced exposures approximately 11 times that achieved with MRHD and greater (on a plasma area under the curve [AUC] basis with maternal subcutaneous doses of 15, 50, or 150 mg/kg twice weekly). ILARIS did not elicit any evidence of embryotoxicity or fetal malformations. There were increases in the incidence of incomplete ossification of the terminal caudal vertebra and misaligned and/or bipartite vertebra in fetuses at all dose levels when compared to concurrent controls suggestive of delay in skeletal development in the marmoset. Since ILARIS does not cross-react with mouse or rat IL-1β, pregnant mice were subcutaneously administered a murine analog of ILARIS at doses of 15, 50, or 150 mg/kg during the period of organogenesis on gestation days 6, 11, and 17. The incidence of incomplete ossification of the parietal and frontal skull bones of fetuses was increased in a dose-dependent manner at all dose levels tested.
8.2 Lactation
Risk Summary
There is no information regarding the presence of canakinumab in human milk or the effects on milk production. There are a small number of published case reports that do not establish an association between maternal canakinumab use during lactation and adverse effects on breastfed infants. Maternal IgG is known to be present in human milk. The effects of canakinumab in breast milk and possible systemic exposure in the breastfed infant are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ILARIS and any potential adverse effects on the breastfed infant from ILARIS or from the underlying maternal condition.
8.4 Pediatric Use
The CAPS trials with ILARIS included a total of 23 pediatric patients with an age range from 4 years to 17 years (11 adolescents were treated subcutaneously with 150 mg, and 12 children were treated with 2 mg/kg based on body weight greater than or equal to 15 kg and less than or equal to 40 kg). The majority of patients achieved improvement in clinical symptoms and objective markers of inflammation (e.g., Serum Amyloid A [SAA] and C-Reactive Protein). Overall, the efficacy and safety of ILARIS in pediatric and adult patients were comparable. Infections of the upper respiratory tract were the most frequently reported infection. The safety and effectiveness of ILARIS in CAPS patients less than 4 years of age has not been established [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.1)].
The safety and effectiveness of ILARIS in SJIA patients less than 2 years of age have not been established [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.3)].
The TRAPS, HIDS/MKD, and FMF trial included a total of 102 pediatric patients (TRAPS, HIDS/MKD and FMF patients) with an age range from 2 to 17 years who received ILARIS. Overall, there were no clinically meaningful differences in the efficacy, safety and tolerability profile of ILARIS in pediatric patients compared to the overall TRAPS, HIDS/MKD, and FMF populations (comprised of adult and pediatric patients, N = 169). The majority of pediatric patients achieved improvement in clinical symptoms and objective markers of inflammation [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.2)].
The safety and effectiveness of ILARIS for the treatment of gout flares in the pediatric population have not been established.
Because IL-1 blockade may interfere with immune response to infections, it is recommended that prior to initiation of therapy with ILARIS, pediatric patients receive all recommended vaccinations. Avoid use of live virus vaccines concurrently with ILARIS treatment in pediatric patients or in infants exposed in utero following maternal administration [see Warnings and Precautions (5.4) and Use in Specific Populations (8.1)].
8.5 Geriatric Use
Clinical studies of ILARIS in patients with CAPS, TRAPS, HIDS/MKD, FMF and Still’s disease, did not include sufficient numbers of patients 65 years and older to determine whether they respond differently from younger patients.
In the clinical studies for gout flares, of the total number of ILARIS- treated patients (n=491), 85 (17.3 %) were 65 years of age and older, while 16 (3.3 %) were 75 years of age and older [see Clinical Studies (14.4)]. Overall, the efficacy profile of ILARIS in patients with gout flares was comparable between the age group of patients less than 65 years of age, and of patients 65 to 75 years of age. The studies did not include sufficient numbers of patients 75 years and older to determine whether they respond differently from younger patients. No new safety findings were observed in patients across these age groups [see Adverse Reactions (6.1)].
- 10 OVERDOSAGE
-
11
DESCRIPTION
Canakinumab is a recombinant, human anti-human-IL-1β monoclonal antibody that belongs to the IgG1/κ isotype subclass. It is expressed in a murine Sp2/0-Ag14 cell line and comprised of two 447- (or 448-) residue heavy chains and two 214-residue light chains, with a molecular mass of 145157 Daltons when deglycosylated. Both heavy chains of canakinumab contain oligosaccharide chains linked to the protein backbone at asparagine 298 (Asn 298).
The biological activity of canakinumab is measured by comparing its inhibition of IL-1β-dependent expression of the reporter gene luciferase to that of a canakinumab internal reference standard, using a stably transfected cell line.
ILARIS Injection
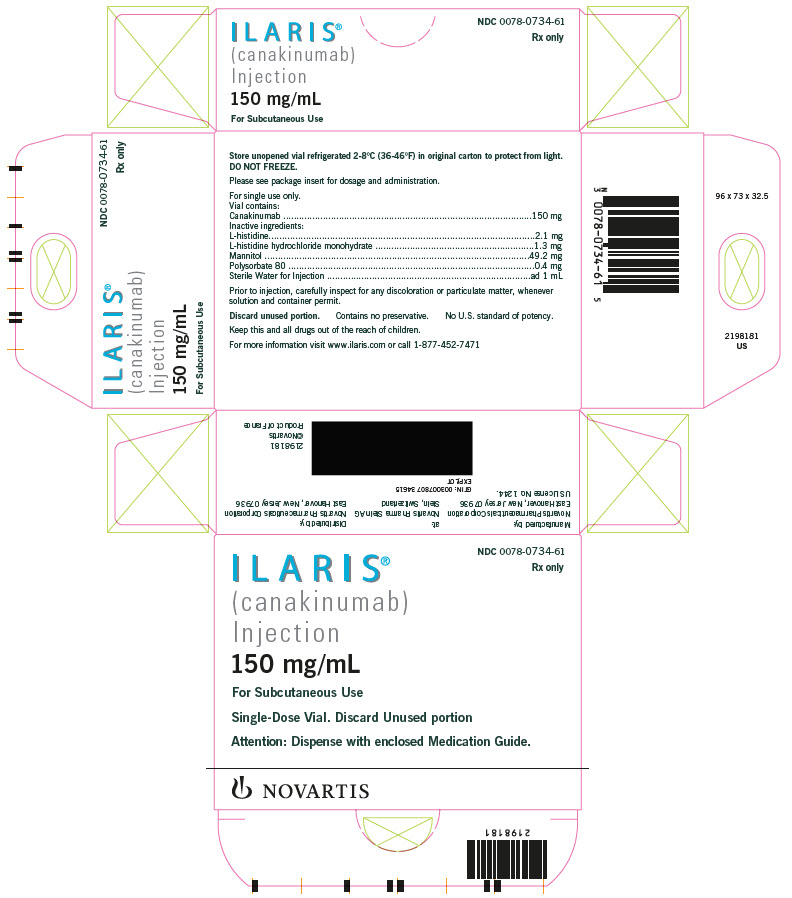
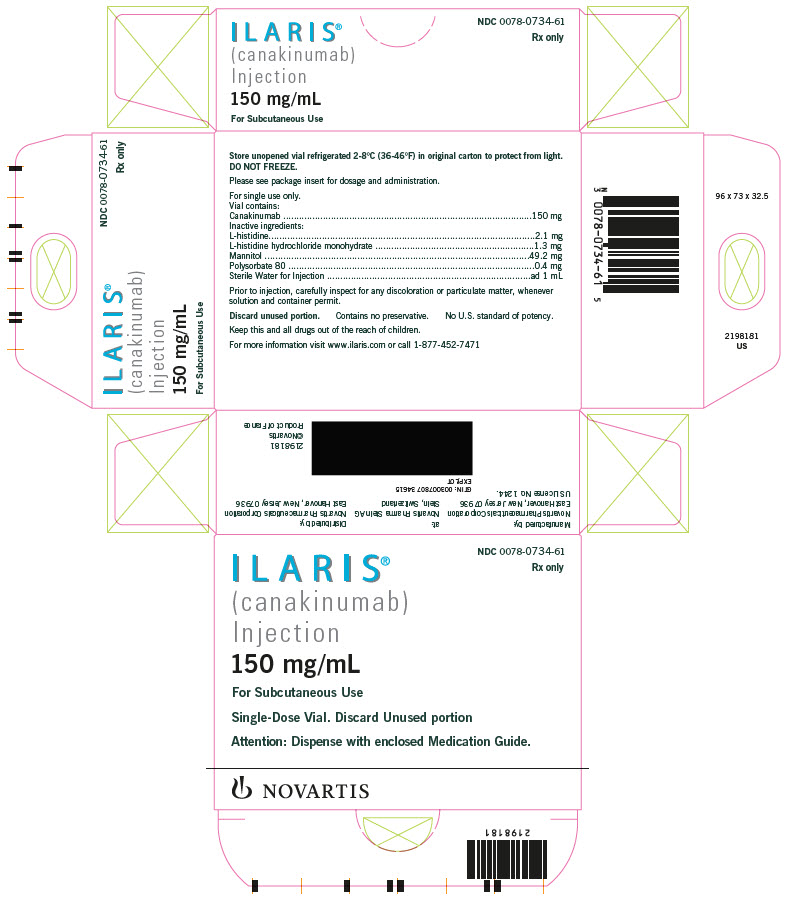
ILARIS (canakinumab) Injection is supplied as a sterile, preservative-free, clear to opalescent, colorless to slightly brownish-yellow solution for subcutaneous injection in a single-dose, glass vial with coated stopper and aluminum flip-off cap. Each vial delivers 1 mL containing 150 mg canakinumab, L-histidine (2.1 mg), L-histidine HCl monohydrate (1.3 mg), mannitol (49.2 mg), polysorbate 80 (0.4 mg), and Sterile Water for Injection.
-
12
CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Canakinumab is a human monoclonal anti-human IL-1β antibody of the IgG1/κ isotype. Canakinumab binds to human IL-1β and neutralizes its activity by blocking its interaction with IL-1 receptors, but it does not bind IL-1α or IL-1 receptor antagonist (IL-1ra).
CAPS refer to rare genetic syndromes generally caused by mutations in the NLRP-3 [nucleotide-binding domain, leucine rich family (NLR), pyrin domain containing 3] gene (also known as Cold-Induced Autoinflammatory Syndrome-1 [CIAS1]). CAPS disorders are inherited in an autosomal dominant pattern with male and female offspring equally affected. Features common to all disorders include fever, urticaria-like rash, arthralgia, myalgia, fatigue, and conjunctivitis.
The NLRP3 protein is an important component of the inflammasome and regulates the protease caspase-1 and controls the activation of IL-1β. Mutations in NLRP3 result in an overactive inflammasome resulting in excessive release of activated IL-1β that drives inflammation. Still’s disease is a severe autoinflammatory disease, driven by innate immunity by means of proinflammatory cytokines such as IL-1β.
Gout flares are characterized by activation of resident macrophages and infiltrating neutrophils in the joint, and concomitant overproduction of IL-1β resulting in an acute painful inflammatory response. IL-1β production by macrophages is triggered by uric acid (monosodium urate monohydrate) crystals in the joint and surrounding tissue through activation of the NLRP3 inflammasome complex.
12.2 Pharmacodynamics
C-reactive protein and Serum Amyloid A (SAA) are indicators of inflammatory disease activity that are elevated in patients with CAPS and gout flares. Elevated SAA has been associated with the development of systemic amyloidosis in patients with CAPS. Following ILARIS treatment, CRP, and SAA levels normalize within 8 days.
In patients with gout flares, CRP and SAA were rapidly reduced following ILARIS treatment. Reductions in CRP and SAA were sustained throughout the 24-week observation period. Improvement in pharmacodynamic markers may not be representative of clinical response.
In SJIA the median percent reduction in CRP from baseline to Day 15 was 91%. Improvement in pharmacodynamic markers may not be representative of clinical response.
12.3 Pharmacokinetics
The pharmacokinetic properties of canakinumab are typical for an IgG-type antibody and are comparable in different diseases but influenced by body weight.
Absorption
The peak serum canakinumab concentration (Cmax) of 16 ± 3.5 mcg/mL occurred approximately 7 days after subcutaneous administration of a single, 150 mg dose subcutaneously to adult CAPS patients. The mean terminal half-life was 26 days. The absolute bioavailability of subcutaneous canakinumab was estimated to be 66%. Exposure parameters (such as AUC and Cmax) increased in proportion to dose over the dose range of 0.30 to 10 mg/kg given as intravenous infusion or from 150 to 300 mg as subcutaneous injection.
Distribution
Canakinumab binds to serum IL-1β. Canakinumab volume of distribution (Vss) varied according to body weight and was estimated to be 6.01 liters in a typical CAPS patient weighing 70 kg, 3.2 liters in a SJIA patient weighing 33 kg, and 6.34 liters for a Periodic Fever Syndrome (TRAPS, HIDS/MKD, FMF) patient weighing 70 kg and 7.9 liters in a typical patient with gout flares of body weight 93 kg. The expected accumulation ratio was 1.3-fold for CAPS patients, 1.6-fold for SJIA patients, and 1.1-fold for patients with gout flares following 6 months of subcutaneous dosing of 150 mg ILARIS every 8 weeks, 4 mg/kg every 4 weeks, and 150 mg every 12 weeks, respectively.
Elimination
Clearance (CL) of canakinumab varied according to body weight and was estimated to be 0.174 L/day in a typical CAPS patient weighing 70 kg, 0.11 L/day in an SJIA patient weighing 33 kg, and 0.17 L/day in a Periodic Fever Syndrome (TRAPS, HIDS/MKD, FMF) patient weighing 70 kg and 0.23 L/day in a typical patient with gout flares of body weight 93 kg. There was no indication of accelerated clearance or time-dependent change in the pharmacokinetic properties of canakinumab following repeated administration. No gender- or age-related pharmacokinetic differences were observed after correction for body weight.
Specific Populations
Pediatric Patients
Pharmacokinetic properties are similar in Periodic Fever Syndromes (CAPS, TRAPS, HIDS/MKD, FMF) and SJIA pediatric populations. In patients less than 2 years of age (n = 7), the exposure of canakinumab were comparable to older age groups with the same weight-based dose.
In CAPS patients, peak concentrations of canakinumab occurred between 2 to 7 days following single subcutaneous administration of ILARIS 150 mg or 2 mg/kg in pediatric patients. The terminal half-life ranged from 22.9 to 25.7 days, similar to the pharmacokinetic properties observed in adults.
In SJIA, exposure parameters (such as AUC and Cmax) were comparable across age groups from 2 years of age and above following subcutaneous administration of canakinumab 4 mg/kg every 4 weeks. Across the indications, SJIA and AOSD, the pharmacokinetics of canakinumab are similar.
In TRAPS, HIDS/MKD, and FMF exposure parameter trough concentrations were comparable across age groups from 2 to less than 20 years following subcutaneous administration of canakinumab 2 mg/kg every 4 weeks.
-
13
NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of canakinumab.
As canakinumab does not cross-react with rodent IL-1β, male and female fertility were evaluated in a mouse model using a murine analog of canakinumab. Male mice were treated weekly beginning 4 weeks prior to mating and continuing through 3 weeks after mating. Female mice were treated weekly for 2 weeks prior to mating through gestation day 3 or 4. The murine analog of canakinumab did not alter either male or female fertility parameters at subcutaneous doses up to 150 mg/kg.
-
14
CLINICAL STUDIES
14.1 Treatment of CAPS
The efficacy and safety of ILARIS for the treatment of CAPS was demonstrated in CAPS Study (NCT00465985), a 3-part trial in patients 9 to 74 years of age with the MWS phenotype of CAPS. Throughout the trial, patients weighing more than 40 kg received ILARIS 150 mg and patients weighing 15 to 40 kg received 2 mg/kg. Part 1 was an 8-week open-label, single-dose period where all patients received ILARIS. Patients who achieved a complete clinical response and did not relapse by Week 8 were randomized into Part 2, a 24-week randomized, double-blind, placebo-controlled withdrawal period. Patients who completed Part 2 or experienced a disease flare entered Part 3, a 16-week open-label active treatment phase. A complete response was defined as ratings of minimal or better for physician’s assessment of disease activity (PHY) and assessment of skin disease (SKD) and had serum levels of C-Reactive Protein (CRP) and Serum Amyloid A (SAA) less than 10 mg/L. A disease flare was defined as a CRP and/or SAA values greater than 30 mg/L and either a score of mild or worse for PHY or a score of minimal or worse for PHY and SKD.
In Part 1, a complete clinical response was observed in 71% of patients one week following initiation of treatment and in 97% of patients by Week 8 (see Table 4 and Figure 1). In the randomized withdrawal period, a total of 81% of the patients randomized to placebo flared as compared to none (0%) of the patients randomized to ILARIS. The 95% confidence interval for treatment difference in the proportion of flares was 53% to 96%. At the end of Part 2, all 15 patients treated with ILARIS had absent or minimal disease activity and skin disease (see Table 4).
In a second trial (NCT00465985), patients 4 to 74 years of age with both MWS and FCAS phenotypes of CAPS were treated in an open-label manner. Treatment with ILARIS resulted in clinically significant improvement of signs and symptoms and in normalization of high CRP and SAA in a majority of patients within 1 week.
Table 4: Physician’s Global Assessment of Auto Inflammatory Disease Activity and Assessment of Skin Disease: Frequency Table and Treatment Comparison in Part 2 (Using LOCF, ITT Population) ILARIS
N = 15Placebo
N = 16
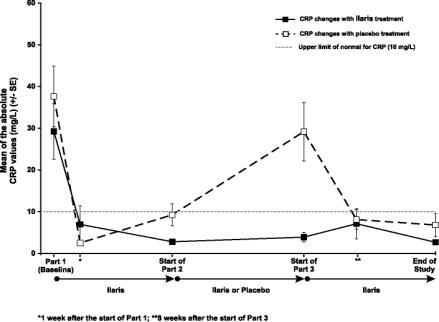
BaselineStart of Part 2 (Week 8) End of Part 2 Start of Part 2 (Week 8) End of Part 2 Physician's Global Assessment of Auto Inflammatory Disease Activity – n (%) Absent 0/31 (0) 9/15 (60) 8/15 (53) 8/16 (50) 0/16 (0) Minimal 1/31 (3) 4/15 (27) 7/15 (47) 8/16 (50) 4/16 (25) Mild 7/31 (23) 2/15 (13) 0/15 (0) 0/16 (0) 8/16 (50) Moderate 19/31 (61) 0/15 (0) 0/15 (0) 0/16 (0) 4/16 (25) Severe 4/31 (13) 0/15 (0) 0/15 (0) 0/16 (0) 0/16 (0) Assessment of skin disease – n (%) Absent 3/31 (10) 13/15 (87) 14/15 (93) 13/16 (81) 5/16 (31) Minimal 6/31 (19) 2/15 (13) 1/15 (7) 3/16 (19) 3/16 (19) Mild 9/31 (29) 0/15 (0) 0/15 (0) 0/16 (0) 5/16 (31) Moderate 12/31 (39) 0/15 (0) 0/15 (0) 0/16 (0) 3/16 (19) Severe 1/32 (3) 0/15 (0) 0/15 (0) 0/16 (0) 0/16 (0) Markers of inflammation CRP and SAA normalized within 8 days of treatment in the majority of patients. Normal mean CRP (Figure 1) and SAA values were sustained throughout CAPS Study 1 in patients continuously treated with canakinumab. After withdrawal of canakinumab in Part 2, CRP (Figure 1) and SAA values again returned to abnormal values and subsequently normalized after reintroduction of canakinumab in Part 3. The pattern of normalization of CRP and SAA was similar.
Figure 1. Mean C-Reactive Protein Levels at the End of Parts 1, 2, and 3 of CAPS Study 1
14.2 Treatment of Periodic Fever Syndromes: TRAPS, HIDS/MKD, and FMF
The efficacy and safety of ILARIS for the treatment of TRAPS, HIDS/MKD, and FMF was demonstrated in a 4-Part study (TRAPS, HIDS/MKD, and FMF Study 1) (NCT02059291) consisting of three separate, disease cohorts (TRAPS, HIDS/MKD, and FMF) which enrolled 185 patients aged greater than 28 days. Patients in each cohort entered a 12-week screening period (Part 1) during which they were evaluated for the onset of disease flare. Patients aged 2 to 76 years were then randomized at flare onset into a 16-week double-blind, placebo-controlled treatment period (Part 2) where they received either 150 mg ILARIS (2 mg/kg for patients weighing less than or equal to 40 kg) subcutaneously or placebo every 4 weeks. Part 3 and Part 4 of this study are ongoing.
Randomized patients in Part 2 treated with ILARIS whose disease flare did not resolve, or who had persistent disease activity from Day 8 up to Day 14 (Physician’s Global Assessment [PGA] greater than or equal to 2 or C-reactive Protein [CRP] greater than 10 mg/L and no reduction by at least 40% from baseline) received an additional dose of 150 mg (or 2 mg/kg for patients weighing less than or equal to 40 kg). Patients treated with ILARIS whose disease flare did not resolve, or who had persistent disease activity from Day 15 up to Day 28 (PGA greater than or equal to 2 or CRP greater than 10 mg/L and no reduction by at least 70% from baseline), also received an additional dose of 150 mg (or 2 mg/kg for patients weighing less than or equal to 40 kg). On or after Day 29, patients treated with ILARIS in Part 2 with PGA greater than or equal to 2 and CRP greater than or equal to 30 mg/L were also up-titrated. All up-titrated patients remained at the increased dose of 300 mg (or 4 mg/kg for patients weighing less than or equal to 40 kg) every 4 weeks.
The primary efficacy endpoint of the randomized, 16-week treatment period (Part 2) was the proportion of complete responders within each cohort as defined by patients who had resolution of their index disease flare at Day 15 and did not experience a new disease flare during the remainder of the 16-week treatment period. Resolution of the index disease flare (initial flare at the time of the randomization) was defined at the Day 15 visit as a PGA Disease Activity score less than 2 (“minimal or no disease”) and C-reactive Protein (CRP) within normal range (less than or equal to 10 mg/L) or reduction greater than or equal to 70% from baseline. The key signs and symptoms assessed in the PGA for each condition were the following: TRAPS: abdominal pain, skin rash, musculoskeletal pain, eye manifestations; HIDS/MKD: abdominal pain; lymphadenopathy, aphthous ulcers; FMF: abdominal pain, skin rash, chest pain, arthralgia/arthritis. A new flare was defined as a PGA score greater than or equal to 2 (“mild, moderate, or severe disease”) and CRP greater to or equal than 30 mg/L. In the 16-week treatment period (Part 2), patients who needed dose escalation, who crossed over from placebo to ILARIS, or who discontinued from the study due to any reason prior to Week 16 were considered as non-responders.
Patients randomized in the TRAPS cohort (N = 46) were aged 2 to 76 years (median age at baseline: 15.5 years) and of this population, 57.8% did not have fever at baseline. Randomized TRAPS patients were those with chronic or recurrent disease activity defined as 6 flares per year (median number of flares per year: 9.0) with PGA greater than or equal to 2 and CRP greater than 10 mg/L (median CRP at baseline: 112.5 mg/L). In the TRAPS cohort, 11/22 (50.0%) patients randomized to ILARIS 150 mg every 4 weeks received up-titration to 300 mg every 4 weeks during the 16-week treatment period, while 21/24 (87.5%) patients randomized to placebo crossed over to ILARIS.
Patients randomized in the HIDS/MKD cohort (N = 72) were aged 2 to 47 years (median age at baseline: 11.0 years) and of this population, 41.7% did not have fever at baseline. Randomized HIDS/MKD patients were those with a confirmed diagnosis of HIDS according to known genetic MVK/enzymatic (MKD) findings, and documented prior history of greater than or equal to 3 febrile acute flares within a 6-month period (median number of flares per year: 12.0) when not receiving prophylactic treatment and during the study, had active HIDS flares defined as PGA greater than or equal to 2 and CRP greater than 10 mg/L (median CRP at baseline: 113.5 mg/L). In the HIDS/MKD cohort, 19/37 (51.4%) patients randomized to ILARIS 150 mg every 4 weeks received up-titration to 300 mg every 4 weeks during the 16-week treatment period, while 31/35 (88.6%) patients randomized to placebo crossed over to ILARIS.
Patients randomized in the FMF cohort (N = 63) were aged 2 to 69 years (median age at baseline: 18.0 years) and of this population, 76.2% did not have fever at baseline. Randomized FMF patients were those with documented active disease despite colchicine therapy or documented intolerance to effective doses of colchicine. Patients had active disease defined as at least one flare per month (median number of flares per year: 18.0) and CRP greater than 10 mg/L (median CRP at baseline: 94.0 mg/L). Patients were allowed to continue their stable dose of colchicine without change. Of the 63 randomized patients, 55 (87.3%) were taking concomitant colchicine therapy on or after randomization. In the FMF cohort, 10/31 (32.3%) patients randomized to ILARIS 150 mg every 4 weeks received up-titration to 300 mg every 4 weeks during the 16-week treatment period, while 27/32 (84.4%) patients randomized to placebo crossed over to ILARIS.
For the primary efficacy endpoint, ILARIS was superior to placebo in the proportion of TRAPS, HIDS/MKD, and FMF patients who resolved their index disease flare at Day 15 and had no new flare over the 16 weeks of treatment from the time of the resolution of the index flare (see Table 5).
Table 5: Proportion of TRAPS, HIDS/MKD, and FMF Patients Who Achieved a Complete Response (Resolution of Index Flare by Day 15 and Maintained Through Week 16) ILARIS 150 mg Placebo Treatment comparison Cohort n/N (%) n/N (%) Odds ratio
95% CIp-value TRAPS 10/22 (45.5%) 2/24 (8.3%) 9.17
(1.51, 94.61)0.005 HIDS/MKD 13/37 (35.1%) 2/35 (5.7%) 8.94
(1.72, 86.41)0.002 FMF 19/31 (61.3%) 2/32 (6.3%) 23.75
(4.38, 227.53)<0.0001 Abbreviation: CI, confidence interval.
n = number of patients with the response.
N = number of patients evaluated for that response in each cohort.At Day 15, a higher proportion of ILARIS-treated patients compared to placebo-treated patients experienced resolution of their index flare in all disease cohorts (see Table 6).
Table 6: Resolution of Index Flare (Full Analysis Set) Resolution at Day 15* ILARIS 150 mg every 4 weeks Placebo Variable n/N (%) n/N (%) TRAPS 14/22 (63.6%) 5/24 (20.8%) HIDS/MKD 24/37 (64.9%) 13/35 (37.1%) FMF 25/31 (80.7%) 10/32 (31.3%) n = number of patients with the response.
N = number of patients evaluated for that response in each cohort.
*Resolution of index disease flare (PGA less than 2 and CRP less than or equal to 10 mg/L or reduction greater than or equal to 70% from baseline).There was supportive evidence of efficacy for ILARIS at Day 15, as compared to placebo, for the components of the primary endpoint, CRP and PGA Disease Activity score, as well as for the secondary endpoint SAA level (see Table 7).
Table 7: Proportion of TRAPS, HIDS/MKD, and FMF Patients Achieving PGA Less Than 2, CRP Less Than or Equal to 10 mg/L and SAA Less Than or Equal to 10 mg/L at Day 15* TRAPS HIDS/MKD FMF Variable ILARIS
150 mgPlacebo Treatment comparison ILARIS
150 mgPlacebo
Treatment comparison ILARIS
150 mgPlacebo
Treatment comparison n/N (%) n/N (%) Odds ratio
95% CIn/N (%) n/N (%) Odds ratio
95% CIn/N (%) n/N (%) Odds ratio
95% CIPGA less than 2 14/22 (63.6%) 8/24 (33.3%) 4.06 (1.12, 14.72) 26/37 (70.3%) 14/35 (40.0%) 3.42 (1.28, 9.16) 27/31 (87.1%) 13/32 (40.6%) 10.07 (2.78, 36.49) CRP less than or equal to 10 mg/L 13/22 (59.1%) 8/24 (33.3%) 3.88 (1.05, 14.26) 25/37 (67.6%) 9/35 (25.7%) 6.05 (2.14, 17.12) 28/31 (90.3%) 9/32 (28.1%) 22.51 (5.41, 93.62) SAA less than or equal to 10 mg/L 7/22 (31.8%) 2/24 (8.3%) 5.06 (0.92, 27.91) 10/37 (27.0%) 4/35 (11.4%) 2.94 (0.82, 10.53) 13/31 (41.9%) 5/32 (15.6%) 3.73 (1.11, 12.52) Abbreviation: CI: confidence interval.
n = number of patients with the response.
N = number of patients evaluated for that response in each cohort.
*ILARIS-treated patients who up-titrated or discontinued prior to Day 15 and placebo-treated patients who switched over to ILARIS or discontinued prior to Day 15 were classified as nonresponders.14.3 Treatment of Still’s Disease: AOSD and SJIA
SJIA
The efficacy of ILARIS for the treatment of active SJIA was assessed in 2 Phase 3 studies (SJIA Study 1 and SJIA Study 2). Patients enrolled were aged 2 to less than 20 years (mean age at baseline: 8.5 years) with a confirmed diagnosis of SJIA at least 2 months before enrollment (mean disease duration at baseline: 3.5 years). Patients had active disease defined as greater than or equal to 2 joints with active arthritis (mean number of active joints at baseline: 15.4), documented spiking, intermittent fever (body temperature greater than 38°C) for at least 1 day within 1 week before study drug administration, and CRP greater than 30 mg/L (normal range less than 10 mg/L) (mean CRP at baseline: 200.5 mg/L). Patients were allowed to continue their stable dose of methotrexate, corticosteroids, and/or NSAIDs without change, except for tapering of the corticosteroid dose as per study design in SJIA Study 2 (see below).
SJIA Study 1 (NCT00886769) was a randomized, double-blind, placebo-controlled, single-dose 4-week study assessing the short-term efficacy of ILARIS in 84 patients randomized to receive a single subcutaneous dose of 4 mg/kg ILARIS or placebo (43 patients received ILARIS and 41 patients received placebo). The primary objective of this study was to demonstrate the superiority of ILARIS versus placebo in the proportion of patients who achieved at least 30% improvement in an adapted pediatric American College of Rheumatology (ACR) response criterion which included both the pediatric ACR core set (ACR30 response) and absence of fever (temperature less than or equal to 38°C in the preceding 7 days) at Day 15.
Pediatric ACR responses are defined by achieving levels of percentage improvement (30%, 50%, and 70%) from baseline in at least 3 of the 6 core outcome variables, with worsening of greater than or equal to 30% in no more than one of the remaining variables. Core outcome variables included a physician global assessment of disease activity, parent or patient global assessment of well-being, number of joints with active arthritis, number of joints with limited range of motion, CRP, and functional ability (Childhood Health Assessment Questionnaire-CHAQ).
Percentages of patients by pediatric ACR response are presented in Table 8.
Table 8: Pediatric ACR Response at Days 15 and 29 Day 15 Day 29 ILARIS Placebo Weighted Difference1 (95% CI)2 ILARIS Placebo Weighted difference1 (95% CI)2 N = 43 N = 41 N = 43 N = 41 ACR30 84% 10% 70% (56%, 84%) 81% 10% 70% (56%, 84%) ACR50 67% 5% 65% (50%, 80%) 79% 5% 76% (63%, 88%) ACR70 60% 2% 64% (49%, 79%) 67% 2% 67% (52%, 81%) 1Weighted difference is the difference between the ILARIS and placebo response rates, adjusted for the stratification factors (number of active joints, previous response to anakinra, and level of oral corticosteroid use).
2CI = confidence interval for the weighted difference.
N = Number of patients.Results for the components of the pediatric ACR core set were consistent with the overall ACR response results, for systemic and arthritic components, including the reduction in the total number of active joints and joints with limited range of motion. Among the patients who returned for a Day 15 visit, the mean change in patient pain score (0 to 100 mm visual analogue scale) was -50.0 mm on ILARIS (N = 43), as compared to +4.5 mm on placebo (N = 25). The mean change in pain score among ILARIS-treated patients was consistent through Day 29. All patients treated with ILARIS had no fever at Day 3 compared to 87% of patients treated with placebo.
SJIA Study 2 (NCT00889863) was a randomized, double-blind, placebo-controlled, withdrawal study of flare prevention by ILARIS in patients with active SJIA. Flare was defined by worsening of greater than or equal to 30% in at least 3 of the 6 core Pediatric ACR response variables combined with improvement of greater than or equal to 30% in no more than 1 of the 6 variables, or reappearance of fever not due to infection for at least 2 consecutive days. The study consisted of 2 major parts: 177 patients were enrolled in the study and received 4 mg/kg ILARIS subcutaneously every 4 weeks in Part I and 100 of these patients continued into Part II to receive either ILARIS 4 mg/kg or placebo subcutaneously every 4 weeks.
Corticosteroid Dose Tapering
Of the total 128 patients taking corticosteroids who entered the open-label portion of Study 2, 92 attempted corticosteroid tapering. Fifty-seven (62%) of the 92 patients who attempted to taper were able to successfully taper their corticosteroid dose and 42 (46%) discontinued corticosteroids.
Time to Flare
Part II was a randomized withdrawal design to demonstrate that the time to flare was longer with ILARIS than with placebo. Follow-up stopped when 37 events had been observed resulting in patients being followed for different lengths of time. The probability of experiencing a flare over time in Part II was statistically lower for the ILARIS treatment group than for the placebo group (Figure 2). This corresponded to a 64% relative reduction in the risk of flare for patients in the ILARIS group as compared to those in the placebo group (hazard ratio of 0.36; 95% CI: 0.17 to 0.75).
Figure 2. Kaplan-Meier Estimates of the Probability to Stay Flare-Free in Part II of SJIA Study 2 by Treatment (ILARIS (ACZ885) and Placebo groups)
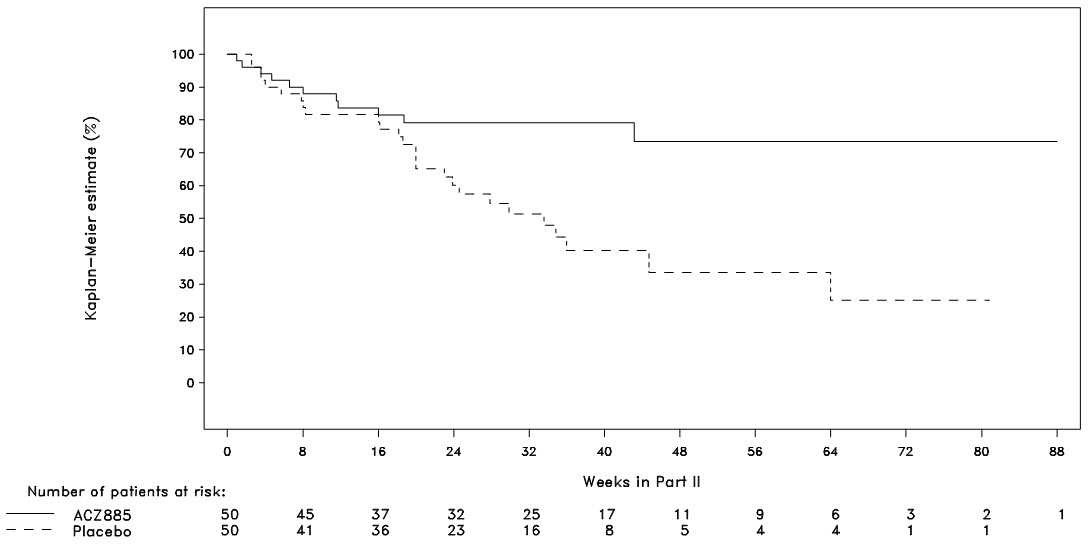
Very few patients were followed for more than 48 weeks.
AOSD
The efficacy of ILARIS in adults with AOSD is based on the pharmacokinetic exposure and extrapolation of the established efficacy of ILARIS in SJIA patients. Efficacy of ILARIS was also assessed in a randomized, double-blind, placebo-controlled study that enrolled 36 patients (22 to 70 years old) diagnosed with AOSD. The efficacy data were generally consistent with the results of a pooled efficacy analysis of SJIA patients.
14.4 Treatment of Gout Flares
The efficacy of ILARIS was demonstrated in two 12-week, randomized, double-blind, active-controlled studies in patients with gout flares for whom NSAIDs and/or colchicine were contraindicated, not tolerated or ineffective, and who had experienced at least three gout flares in the previous year (Studies 1 and 2). The studies continued in 1) two 12-week, double-blind, active-controlled extensions, followed by 2) two open-label extensions and continued 3) in a third open-label extension (combined for both studies) up to a maximum of 36 months where all patients were treated with ILARIS upon a new flare.
In Study 1 (NCT01029652), patients were randomized to receive ILARIS 150 mg subcutaneous (N = 115) or triamcinolone acetonide 40 mg intramuscular (N = 115) at baseline and thereafter treated upon a new flare. Two patients randomized to canakinumab were not included in the analysis as they did not receive any study medication. In Study 2 (NCT01080131), patients were randomized to receive ILARIS 150 mg subcutaneous (N =112) or triamcinolone acetonide 40 mg intramuscular (N =114) at baseline and thereafter treated upon a new flare.
In Studies 1 and 2, over 85% of patients had at least one co-morbidity, including hypertension (60%), obesity (53%), diabetes (15%), and ischemic heart disease (12%). Twenty-five percent of patients had chronic kidney disease (stage ≥ 3), based on eGFR. Concomitant treatment with allopurinol or other uric acid lowering therapies was reported by 42% of patients at entry.
The majority of patients (73%) reported between 3-6 flares in the year prior to study entry and the remainder reported seven or more flares. Approximately one-third of the patients enrolled [76 in the ILARIS group (33.5%) and 84 in the triamcinolone acetonide (36.7%) group] had documented inability (intolerance, contraindication or lack of response) to use both, NSAIDs and colchicine. The remainder had intolerance, contraindication or lack of response to either NSAIDs or colchicine.
In both studies, the co-primary endpoints were: (i) patient’s assessment of gout flare pain intensity at the most affected joint at 72 hours post-dose measured on a 0-100 mm visual analogue scale (VAS) and (ii) the time to first new gout flare. The studies aimed to determine whether ILARIS 150 mg would be superior to triamcinolone acetonide 40 mg.
Study 3 (NCT01356602), an additional 12-week, randomized, double-blind, active-controlled study, enrolled 397 patients with ILARIS 150 mg subcutaneous (Pre-Filled Syringe (PFS), N=133, Lyophilizate (LYO), N=132) or triamcinolone acetonide 40 mg intramuscular (N=132). Eight patients (2 ILARIS PFS, 3 ILARIS LYO, 3 triamcinolone) were not included for efficacy assessment as they did not receive study medication. Pain intensity at the most affected joint, assessed on a 0-100 mm VAS at 72-hours post-dose was the primary endpoint, and time to first new gout flare was a secondary endpoint. Approximately 44% of patients (45.9% ILARIS PFS group, 47.4%, ILARIS LYO group and 40.6% in the triamcinolone acetonide group) were unable to use NSAIDs and colchicine (due to contraindications, intolerance, or inadequate response) in this study.
Analyses of both endpoints were conducted for Studies 1, 2, and 3 for the subpopulation of patients unable to use NSAIDs and colchicine (due to contraindications, intolerance, or inadequate response) and overall population of patients unable to use NSAIDs and/or colchicine.
Efficacy on Pain
In all studies (Study 1, 2, and 3), pain intensity of the most affected joint (0-100 mm VAS) at 72 hours post-dose was consistently lower for patients treated with ILARIS compared with triamcinolone acetonide in the subpopulation of patients unable to use NSAIDs and colchicine as shown in Table 9, and Figure 3 (Study 3). This benefit of ILARIS on pain intensity was comparable to the overall patient populations i.e., patients unable to use NSAIDs and/or colchicine in all three studies (see Table 9).
Table 9: Pain Intensity of the Most Affected Joint at 72-h post treatment Study Population ILARIS
150 mgTriamcinolone
acetonide
40 mgDifference (95% CI)* in
Pain Intensity 72 Hours
Post-dose VAS (0-100 mm):
ILARIS vs. Triamcinolone acetonideN Mean (SE)* N Mean (SE)* Study 1 Patients unable to use NSAIDs and colchicine 22 21.4 (6.05) 37 38.4 (4.65) -17.0 mm
(-32.3, -1.6)Patients unable to use NSAIDs and/or colchicine 113 27.9 (2.42) 115 39.7 (2.40) -11.8 mm
(-18.5, -5.1)Study 2 Patients unable to use NSAIDs and colchicine 53 24.1 (3.32) 44 33.1 (3.65) -9.1 mm
(-18.9, 0.8)Patients unable to use NSAIDs and/or colchicine 112 21.9 (2.31) 114 31.7 (2.29) -9.8 mm
(-16.2, -3.4)Study 3 Patients unable to use NSAIDs and colchicine 62 20.8 (3.11) 51 40.3 (3.42) -19.5 mm
(-28.6, -10.3)60# 18.5 (3.16) -21.8 mm
(-31.0, -12.6)Patients unable to use NSAIDs and/or colchicine 129 19.7 (2.05) 129 32.4 (2.05) -12.7 mm
(-18.4, -7.0)131# 17.0 (2.04) -15.4 mm
(-21.1, -9.8)Abbreviation: CI = confidence interval; SE=Standard Error
#Prefilled Syringe (PFS) formulation.
*Adjusted mean, standard error for mean and difference between treatment groups are estimated based on analysis of covariance (ANCOVA) model with treatment, baseline VAS score and baseline BMI as covariates. For Study 3, the use of urate lowering therapy (Yes/No) at baseline is also included in the model as additional covariate.
N = number of patients randomized and received at least one dose of study treatment.Figure 3. Pain Intensity Over Time in the Subpopulation of Patients Unable to Use NSAIDs and Colchicine (Study 3, ILARIS (ACZ885) 150mg)
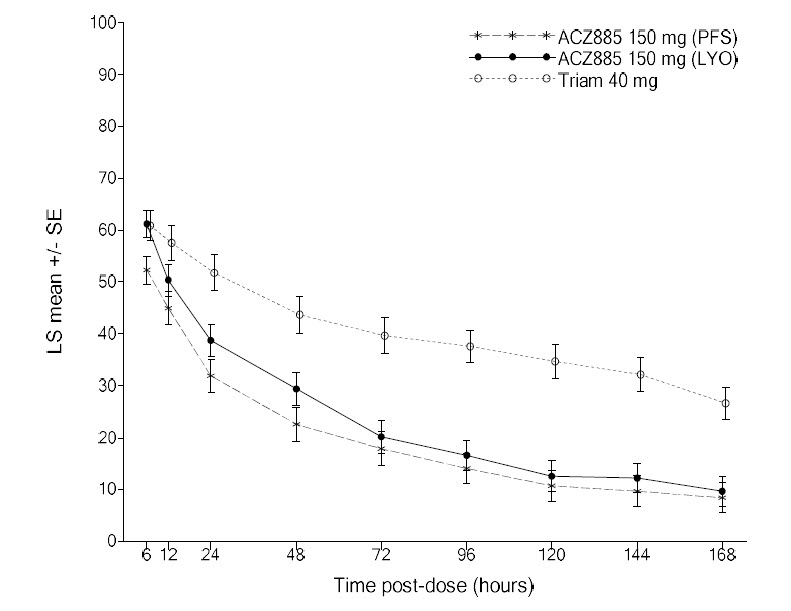
Time to New Flare
In the subpopulation of patients in Studies 1, 2 and 3 unable to use NSAIDs and colchicine, time to new flare over 12 weeks from randomization showed a reduction in the risk of a new flare when treated with ILARIS compared with triamcinolone acetonide 40 mg (see Table 10).
This risk reduction for a new flare after ILARIS treatment versus triamcinolone acetonide was comparable to the overall patient population over 12 weeks in all 3 studies (see Table 10).
Table 10: Time to New Flare Over the 12 Weeks From Randomization Study Population ILARIS
150 mgTriamcinolone
acetonide
40 mgRisk reduction for a new
flare ILARIS vs.
Triamcinolone acetonide
Hazard ratio#
(95% CI)N Flare rate*(n) N Flare rate*(n) Study 1 Patients unable to use NSAIDs and colchicine 22 14% (3) 38 46% (17) 75%
0.25
(0.07, 0.85)Patients unable to use NSAIDs and/or colchicine 113 19% (21) 115 37% (40) 55 %
0.45
(0.26 to 0.76)Study 2 Patients unable to use NSAIDs and colchicine 54 16% (8) 46 43% (19) 72%
0.28
(0.12, 0.65)Patients unable to use NSAIDs and/or colchicine 112 14% (15) 114 38% (42) 68%
0.32
(0.18 to 0.58)Study 3 Patients unable to use NSAIDs and colchicine 62 10% (6) 51 32% (15) 71%
0.29
(0.11, 0.74)60# 3% (2) 91%
0.09
(0.02, 0.41)Patients unable to use NSAIDs and/or colchicine 129 10% (12) 129 44% (52) 82%
0.18
(0.10, 0.34)131# 9% (12) 83%
0.17
(0.09, 0.33)Abbreviation: CI = confidence interval.
#Prefilled Syringe (PFS) formulation.
* Flare rates up to 12 weeks are estimated using Kaplan-Meier method; n = number of patients with new flares. The risk reduction and hazard ratio between treatment groups are estimated using Cox proportional hazard (Cox-PH) model with treatment and baseline BMI as covariates. For study 3, the use of urate lowering therapy (Yes/No) at baseline is also included in the model as additional covariate.
N = number of patients randomized and received at least one dose of study treatment. -
16
HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
ILARIS Injection (Solution)
Carton of 1 vial NDC 0078-0734-61
Each single-dose vial of ILARIS (canakinumab) Injection delivers 150 mg/mL sterile, preservative-free, clear to slightly opalescent, colorless to a slight brownish to yellow solution.
Storage and Handling The unopened vial must be stored refrigerated at 2°C to 8°C (36°F to 46°F). Do not freeze. Store in the original carton to protect from light. Do not use beyond the date stamped on the label. ILARIS does not contain preservatives. Discard any unused portions of ILARIS or waste material in accordance with local requirements.
Keep this and all drugs out of the reach of children.
-
17
PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Advise the patient of the potential benefits and risks of ILARIS. Physicians should instruct their patients to read the Medication Guide before starting ILARIS therapy.
Drug Administration
Advise the patient that healthcare providers should perform administration of ILARIS by the subcutaneous injection route [see Dosage and Administration (2.1)].
Infections
Caution the patient that ILARIS use has been associated with serious infections. Counsel the patient to contact their healthcare professional immediately if they develop an infection after starting ILARIS. Treatment with ILARIS should be discontinued if a patient develops a serious infection. Counsel the patient not to take any IL-1 blocking drug, including ILARIS, if they are also taking a drug that blocks TNF, such as etanercept, infliximab, or adalimumab. Use of ILARIS with other IL-1 blocking agents, such as rilonacept and anakinra is not recommended. Caution the patient not to receive ILARIS if they have a chronic or active infection, including HIV, Hepatitis B, or Hepatitis C [see Warnings and Precautions (5.1)].
Vaccinations
Prior to initiation of therapy with ILARIS, physicians should review with adult and pediatric patients their vaccination history relative to current medical guidelines for vaccine use, including taking into account the potential of increased risk of infection during treatment with ILARIS [see Warnings and Precautions (5.4)].
Injection-Site Reactions
Physicians should explain to patients that a very small number of patients in the clinical trials experienced a reaction at the subcutaneous injection site. Injection-site reactions may include pain, erythema, swelling, pruritus, bruising, mass, inflammation, dermatitis, edema, urticaria, vesicles, warmth, and hemorrhage. Healthcare providers should be cautioned to avoid injecting into an area that is already swollen or red. Any persistent reaction should be brought to the attention of the prescribing physician.
Hypersensitivity Reactions
Counsel the patient to stop taking ILARIS and contact their healthcare provider immediately if they develop serious skin reactions or signs of allergic reaction, such as difficulty breathing or swallowing, nausea, dizziness, skin rash, itching, hives, palpitations, or low blood pressure [see Warnings and Precautions (5.3)].
Pregnancy
Advise female patients of the potential risk to a fetus [see Use in Specific Populations (8.1)].
Manufactured by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936US License Number 1244
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936© Novartis
T2024-80
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: November 2024 Medication Guide
ILARIS® (i-LAHR-us)
(canakinumab)
injection, for subcutaneous useWhat is the most important information I should know about ILARIS?
ILARIS can cause serious side effects, including:
-
Increased risk of serious infections. ILARIS can lower the ability of your immune system to fight infections. Your healthcare provider should:
- test you for tuberculosis (TB) before you receive ILARIS.
- monitor you closely for symptoms of TB during treatment with ILARIS.
- check you for symptoms of any type of infection before, during, and after your treatment with ILARIS.
See "What are possible side effects of ILARIS?" for more information about side effects.What is ILARIS?
ILARIS is a prescription medicine injected by your healthcare provider just below the skin (subcutaneous) used to treat:
- The following Periodic Fever Syndromes:
- Adults and children 4 years of age and older who have auto-inflammatory diseases called Cryopyrin-Associated Periodic Syndromes (CAPS), including:
- Familial Cold Auto-inflammatory Syndrome (FCAS).
- Muckle-Wells Syndrome (MWS).
- Adults and children who have an auto-inflammatory disease called Tumor Necrosis Factor Receptor Associated Periodic Syndrome (TRAPS).
- Adults and children who have an auto-inflammatory disease called Hyperimmunoglobulin D Syndrome (HIDS) [also known as Mevalonate Kinase Deficiency (MKD)].
- Adults and children who have an auto-inflammatory disease called Familial Mediterranean Fever (FMF).
- Adults and children 4 years of age and older who have auto-inflammatory diseases called Cryopyrin-Associated Periodic Syndromes (CAPS), including:
- Still’s disease, including Adult-Onset Still’s Disease (AOSD) and Systemic Juvenile Idiopathic Arthritis (SJIA) in children 2 years of age and older.
- Gout flares in adults who:
- are not able to receive or tolerate treatment with non-steroidal anti-inflammatory drugs (NSAIDS) and colchicine.
- have not responded to treatment with NSAIDS and colchicine.
- are not able to receive repeated treatment with steroids.
- SJIA in children under 2 years of age.
- CAPS in children under 4 years of age.
- gout flares in children.
Who should not receive ILARIS?
- Do not receive ILARIS if you are allergic to canakinumab or any of the ingredients in ILARIS. See the end of this Medication Guide for a complete list of ingredients in ILARIS.
Before you receive ILARIS, tell your healthcare provider about all your medical conditions, including if you: - think you have or are being treated for an active infection.
- have symptoms of an infection.
- have a history of infections that keep coming back.
- have a history of low white blood cells.
- have or have had HIV, Hepatitis B, or Hepatitis C.
- have recently received or are scheduled to receive any vaccinations.
- You should be brought up to date with all age required vaccines before starting treatment with ILARIS.
- You should not receive ‘live’ vaccines while you are being treated with ILARIS and until your healthcare provider tells you that your immune system is no longer weakened.
- are pregnant or planning to become pregnant. It is not known if ILARIS will harm your unborn baby. Tell your healthcare provider right away if you become pregnant while receiving ILARIS.
- received ILARIS while you were pregnant. It is important that you tell your baby’s healthcare provider before any vaccinations are given to your baby within 4 to 12 months after you received your last dose of ILARIS before giving birth.
- are breastfeeding or planning to breastfeed. It is not known if ILARIS passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you receive ILARIS.
- medicines that affect your immune system.
- medicines called IL-1 blocking agents, such as Kineret® (anakinra), Arcalyst® (rilonacept).
- medicines called Tumor Necrosis Factor (TNF) inhibitors, such as Enbrel® (etanercept), Humira® (adalimumab), Remicade® (infliximab), Simponi® (golimumab), or Cimzia® (certolizumab pegol).
- medicines that affect enzyme metabolism.
How should I receive ILARIS?
- ILARIS is given by your healthcare provider as an injection under the skin (subcutaneous injection):
- every 8 weeks for CAPS.
- every 4 weeks for TRAPS, HIDS/MKD, FMF, AOSD, and SJIA.
- as a single dose at the time of a gout flares. If you have a new flare and need another dose of ILARIS, you must wait at least 12 weeks before receiving the next dose.
What are the possible side effects of ILARIS?
ILARIS can cause serious side effects, including:
- See “What is the most important information I should know about ILARIS?”
-
decreased ability of your body to fight infections (immunosuppression). For people treated with medicines that cause immunosuppression like ILARIS, the chances of getting cancer may increase.
- serious allergic reactions. Stop taking ILARIS, contact your healthcare provider, and get emergency help right away if you have any of these symptoms of a serious allergic reaction:
◦ swelling of the lips, tongue, mouth, or face
◦ trouble breathing
◦ wheezing
◦ severe itching◦ skin rash, hives, redness, or swelling outside of the injection site area
◦ dizziness or fainting
◦ fast heartbeat or pounding in your chest (tachycardia)
◦ sweating- risk of infection with live vaccines. You should not get live vaccines if you are receiving ILARIS. Tell your healthcare provider if you are scheduled to receive any vaccines.
When ILARIS is used for the treatment of CAPS:• cold symptoms • headache • feeling like you are spinning (vertigo) • diarrhea • cough • weight gain • flu (influenza) • body aches • injection-site reactions (such as redness, swelling, warmth, or itching) • runny nose • nausea, vomiting, and diarrhea (gastroenteritis) • nausea When ILARIS is used for the treatment of TRAPS, HIDS/MKD, and FMF: • cold symptoms • runny nose • nausea, vomiting, and diarrhea (gastroenteritis) • upper respiratory tract infection • sore throat • injection-site reactions (such as redness, swelling, warmth, or itching) When ILARIS is used for the treatment of Still’s disease (AOSD and SJIA): • cold symptoms • runny nose • nausea, vomiting, and diarrhea (gastroenteritis) • upper respiratory tract infection • sore throat • stomach pain • pneumonia • urinary tract infection • injection-site reactions (such as redness, swelling, warmth, or itching) When ILARIS is used for the treatment of gout flares: • cold symptoms • urinary tract infection • upper respiratory tract infection • increased levels of triglycerides in the blood • back pain Tell your healthcare provider about any side effect that bothers you or does not go away.
These are not all the possible side effects of ILARIS. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of ILARIS.
Medicines are sometimes prescribed for purposes other than those listed in this Medication Guide. You can ask your pharmacist or healthcare provider for information about ILARIS that was written for health professionals.What are the ingredients in ILARIS?
Active ingredient: canakinumab.
Inactive ingredients: Solution for Injection: L-histidine, L-histidine HCl monohydrate, mannitol, polysorbate 80, sterile water for injection.What are Periodic Fever Syndromes?
Periodic Fever Syndromes is the name for several different autoinflammatory diseases, including CAPS, TRAPS, HIDS/MKD, and FMF. People with these diseases cannot keep certain chemicals made by their body (interleukin-1 beta, also called IL-1β) at the correct level. All these diseases have symptoms that often come and go, with irritated body parts (inflammation) and elevated body temperature (fever). These conditions have a dysregulation of IL-1β production and share similar clinical features of recurrent episodes of inflammation and fever, such as rash, headache, pain (mostly in the joints, belly, eyes, muscles), fatigue, inflammation of other organs, such as heart, lungs, spleen, and brain.
What is Still’s Disease (AOSD and SJIA)?
Still’s disease (which is referred to as AOSD in adults and SJIA in children) is an autoinflammatory disorder which can be caused by having too much or being too sensitive to certain proteins, including interleukin-1 beta (IL-1β), and can lead to symptoms such as fever, rash, headache, feeling very tired (fatigue), or painful joints and muscles.
What is Gout Flare?
A gout flare is caused when a chemical called urate builds-up in the body, and needle-shaped crystals form in and around the joint. These crystals lead to inflammation with excessive production of certain proteins, such as interleukin-1 beta (also called IL-1β), which in turn can lead to sudden, severe pain, redness, warmth and swelling in a joint.
What is Macrophage Activation Syndrome (MAS)?
MAS is a syndrome associated with Still’s disease and some other autoinflammatory diseases like HIDS/MKD that can lead to death. Tell your healthcare provider right away if your AOSD or SJIA symptoms get worse or if you have any of these symptoms of an infection:- a fever lasting longer than 3 days.
- a cough that does not go away.
- redness in one part of your body.
- warm feeling or swelling of your skin.
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
US License Number 1244
Distributed by: Novartis Pharmaceuticals Corporation, East Hanover, New Jersey 07936
© Novartis
For more information about ILARIS, call 1-888-669-6682 or visit www.ILARIS.com.
Kineret®, Arcalyst®, Enbrel®, Humira®, Remicade®, Simponi®, and Cimzia® are trademarks of Amgen, Regeneron, Immunex Corporation, AbbVie Biotechnology Ltd., Centocor Ortho Biotech Inc., Janssen Biotech Inc., and the UCB Group of companies, respectively.T2024-81
-
Increased risk of serious infections. ILARIS can lower the ability of your immune system to fight infections. Your healthcare provider should:
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ILARIS
canakinumab injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0078-0734 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CANAKINUMAB (UNII: 37CQ2C7X93) (CANAKINUMAB - UNII:37CQ2C7X93) CANAKINUMAB 150 mg in 1 mL Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) 2.1 mg in 1 mL HISTIDINE MONOHYDROCHLORIDE (UNII: 1D5Q932XM6) 1.3 mg in 1 mL MANNITOL (UNII: 3OWL53L36A) 49.2 mg in 1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.4 mg in 1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0078-0734-61 1 in 1 CARTON 12/22/2016 1 1 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125319 12/22/2016 Labeler - Novartis Pharmaceuticals Corporation (002147023)