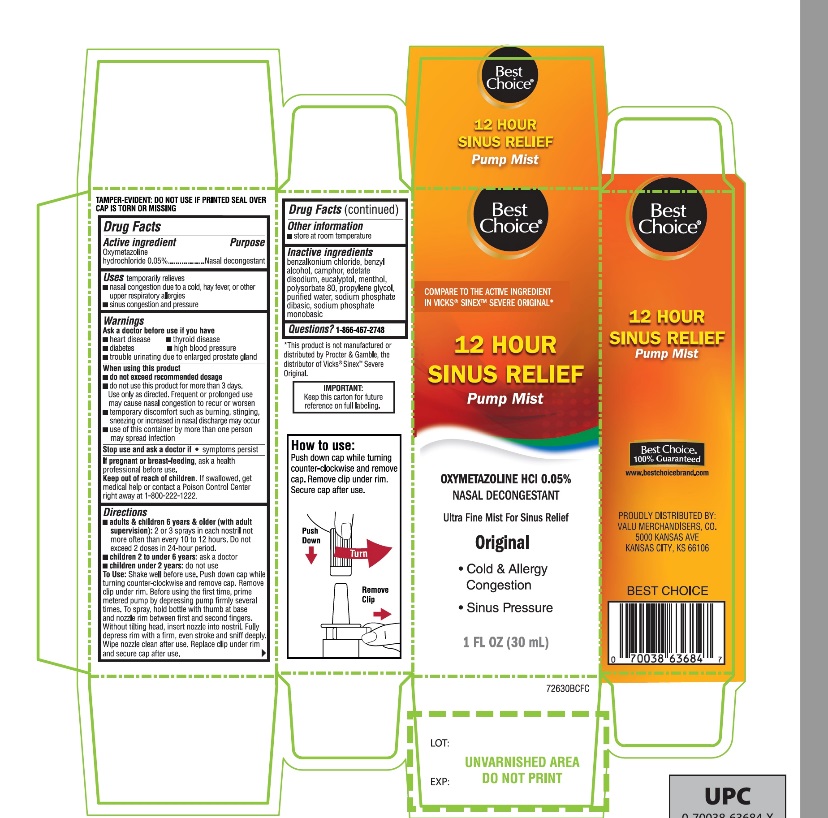
Label: BEST CHOICE ULTRA FINE MIST- oxymetazoline hydrochloride spray
- NDC Code(s): 63941-726-30
- Packager: BEST CHOICE (VALU MERCHANDISERS COMPANY)
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 22, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
- Warnings
- Ask a doctor before use if you have
-
When using this product
- ▪
- do not exceed recommended dosage
- ▪
- do not use this product for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- ▪
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- ▪
- use of this container by more than one person may spread infection
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
Directions
- ▪
- adults & children 6 years & older (with adult supervision): 2 or 3 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- ▪
- Children 2 to under 6 years: ask a doctor
- ▪
- Children 2 years: ask a doctor
Shake well before use. Before using the first time, remove the protective cap from the tip by pressing and twisting off the cap. Rotate the lock tab to align arrow marks to unlock the pump. Prime metered pump by depressing pump firmly several times. To spray, hold bottle with thumb at base and nozzle between first and second fingers without titling head, insert nozzle into nostril. Fully depress rim with a firm, even stroke and sniff deeply. Wipe nozzle clean after use. Lock the pump by ensuring that arrow marks are not aligned and replace the protective cap on tip.
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
Best Choice®
NDC# 63941-726-30
COMPARE TO THE ACTIVE INGREDIENT IN VICKS® SINEX®*
12 HOUR SINUS RELIEF
OXYMETAZOLINE HCl 0.05%
NASAL DECONGESTANT
Ultra-Fine Mist For Sinus Relief
- •
- Cold & allergy congestion
- •
- Sinus pressure
1 FL OZ (30 mL)
IMPORTANT: KEEP THE CARTON FOR FUTURE REFERENCE ON FULL LABELING
Best Choice® 100% Guaranteed
PROUDLY DISTRIBUTED BY:
VALU MERCHANDISERS, CO.
5000 KANSAS AVE
KANSAS CITY, KS 66106
*This product is not manufactured or distributed by Procter and Gamble, the distributer of Vicks® Sinex®
-
INGREDIENTS AND APPEARANCE
BEST CHOICE ULTRA FINE MIST
oxymetazoline hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63941-726 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OXYMETAZOLINE HYDROCHLORIDE (UNII: K89MJ0S5VY) (OXYMETAZOLINE - UNII:8VLN5B44ZY) OXYMETAZOLINE HYDROCHLORIDE 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BENZYL ALCOHOL (UNII: LKG8494WBH) CAMPHOR (NATURAL) (UNII: N20HL7Q941) EDETATE DISODIUM (UNII: 7FLD91C86K) EUCALYPTOL (UNII: RV6J6604TK) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC, UNSPECIFIED FORM (UNII: GR686LBA74) SODIUM PHOSPHATE, MONOBASIC, UNSPECIFIED FORM (UNII: 3980JIH2SW) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63941-726-30 1 in 1 CARTON 05/21/2019 1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 05/21/2019 Labeler - BEST CHOICE (VALU MERCHANDISERS COMPANY) (868703513)