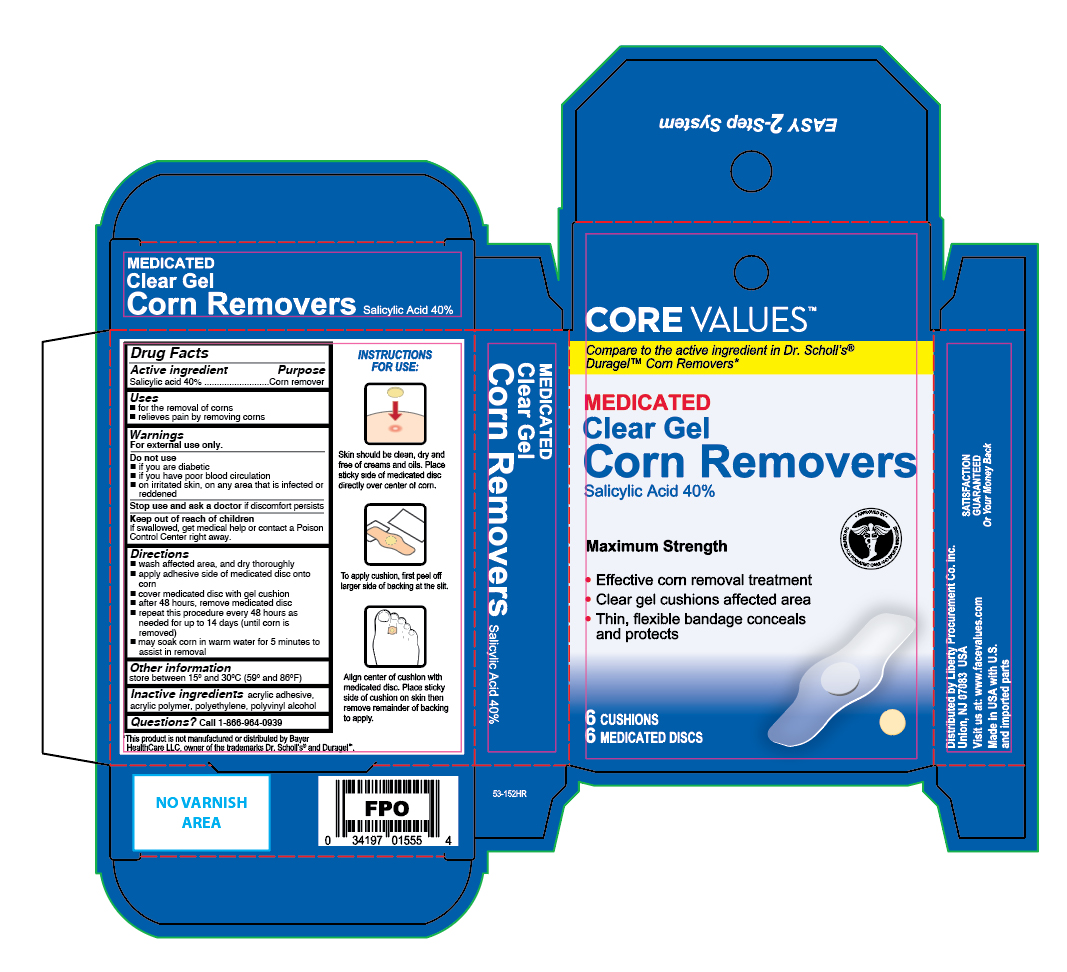
Label: CLEAR GEL CORN REMOVER- salicylic acid patch
- NDC Code(s): 63940-165-06
- Packager: Harmon Store Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
-
Directions
- wash affected area and dry thoroughly
- apply adhesive side down of medicated disc on to the corn
- cover the medicated disc with corn cushion
- after 48 hours, remove medicated disc
- repeat this procedure every 48 hours as needed for up to 14 days (until corn is removed)
- may soak corn in warm water for 5 minutes to assist in removal
- Other Information
- Inactive Ingredients
- Questions
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CLEAR GEL CORN REMOVER
salicylic acid patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63940-165 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 40 mg in 6 Inactive Ingredients Ingredient Name Strength VINYL ACETATE (UNII: L9MK238N77) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63940-165-06 6 in 1 PACKAGE; Type 0: Not a Combination Product 01/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M030 01/01/2018 Labeler - Harmon Store Inc. (804085293)