Label: NORELGESTROMIN AND ETHINYL ESTRADIOL patch
- NDC Code(s): 70771-1777-1, 70771-1777-3
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
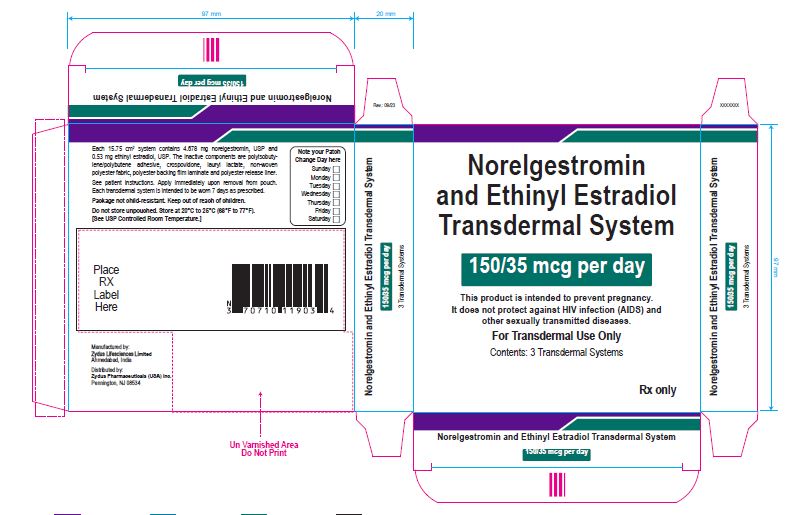
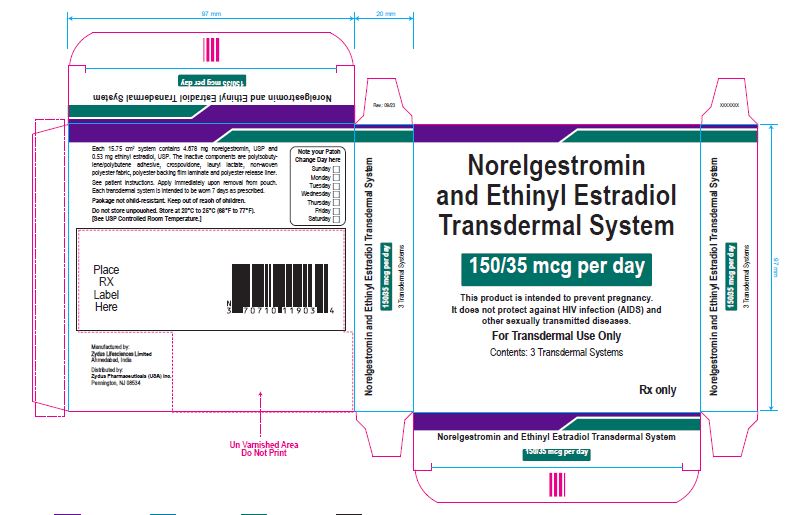
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Norelgestromin and Ethinyl Estradiol Transdermal System 150/35 mcg per day
This product is intended to prevent pregnancy. It does not protect against HIV infection (AIDS) and other sexually transmitted diseases.
For Transdermal Use Only
Contents: 3 Transdermal Systems
Rx only
Each 15.75 cm2 system contains 4.678 mg norelgestromin, USP and 0.53 mg ethinyl estradiol, USP. The inactive components are polyisobutylene/polybutene adhesive, crospovidone, lauryl lactate, non-woven polyester fabric, polyester backing film laminate and polyester release liner.
See patient instructions. Apply immediately upon removal from pouch. Each transdermal system is intended to be worn 7 days as prescribed.
Package not child-resistant. Keep out of reach of children.
Do not store unpouched. Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Manufactured by:
Zydus Lifesciences Ltd.
Ahmedabad, India
Distributed by:
Zydus Pharmaceuticals (USA) Inc.
Pennington, NJ 08534
Rev.: 10/22
-
INGREDIENTS AND APPEARANCE
NORELGESTROMIN AND ETHINYL ESTRADIOL
norelgestromin and ethinyl estradiol patchProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70771-1777 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NORELGESTROMIN (UNII: R0TAY3X631) (NORELGESTROMIN - UNII:R0TAY3X631) NORELGESTROMIN 150 ug in 1 mg ETHINYL ESTRADIOL (UNII: 423D2T571U) (ETHINYL ESTRADIOL - UNII:423D2T571U) ETHINYL ESTRADIOL 35 ug in 1 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (UNII: 2S7830E561) LAURYL LACTATE (UNII: G5SU0BFK7O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70771-1777-3 3 in 1 CARTON 11/21/2023 1 NDC:70771-1777-1 1 in 1 POUCH 1 7 mg in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA214594 11/21/2023 Labeler - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(70771-1777) , MANUFACTURE(70771-1777)