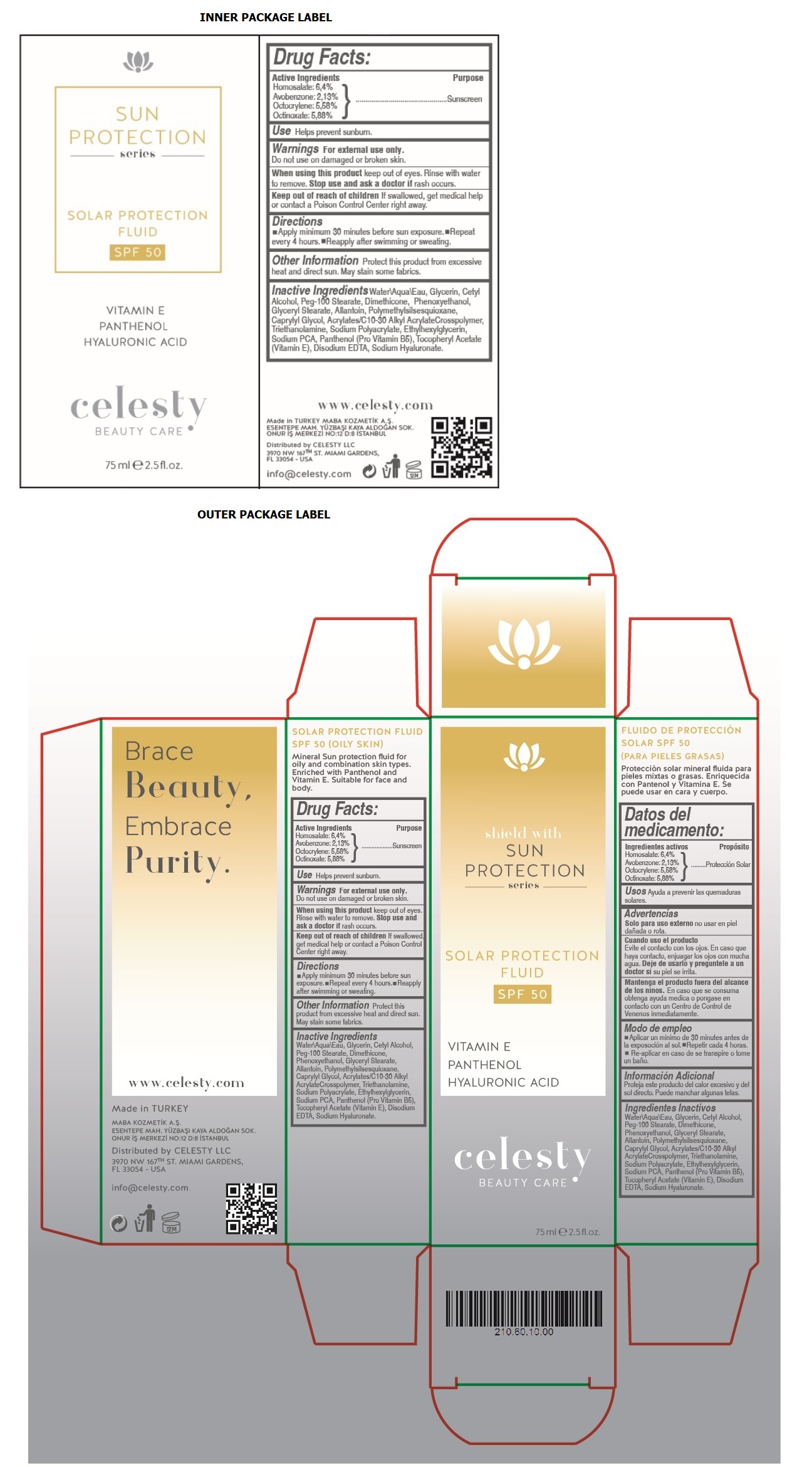
Label: CELESTY SUN PROTECTION SERIES SOLAR PROTECTION FLUID SPF 50- homosalate, avobenzone, octocrylene, octinoxate cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 81120-109-01 - Packager: MABA KOZMETIK LIMITED SIRKETI
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 8, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts:
- Active Ingredients
- Purpose
- Use
- Warnings
- Directions
- Other Information
-
Inactive Ingredients
Water\Aqua\Eau, Glycerin, Cetyl Alcohol, Peg-100 Stearate, Dimethicone, Phenoxyethanol, Glyceryl Stearate, Allantoin, Polymethylsilsesquioxane, Caprylyl Glycol, Acrylates/C 10-30 Alkyl Acrylate Crosspolymer, Triethanolamine, Sodium Polyacrylate, Ethylhexylglycerin, Sodium PCA, Panthenol (Pro Vitamin B5), Tocopheryl Acetate (Vitamin E), Disodium EDTA, Sodium Hyaluronate.
-
SPL UNCLASSIFIED SECTION
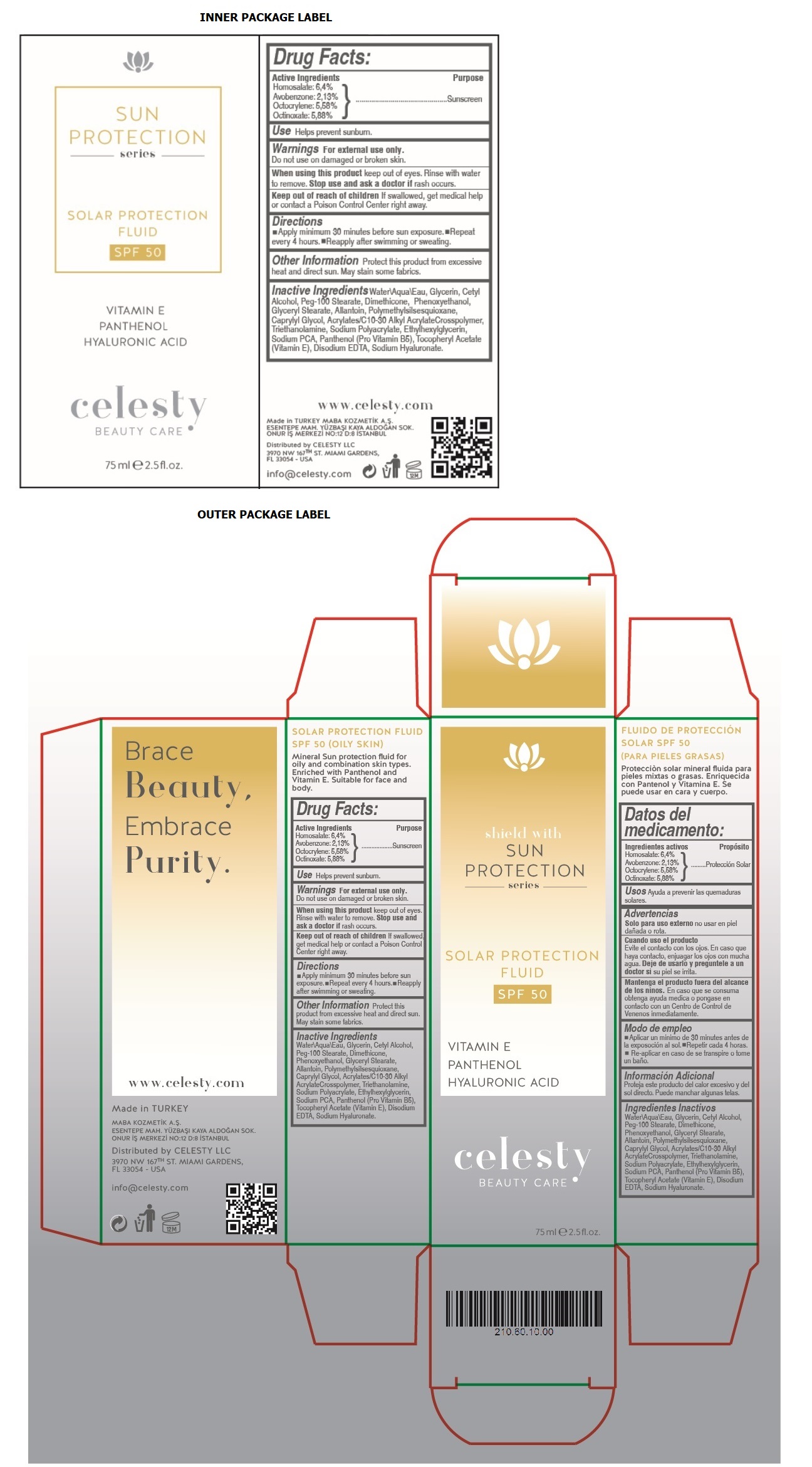
shield with SUN PROTECTION series
VITAMIN E
PANTHENOL
HYALURONIC ACID
BEAUTY CARE
SOLAR PROTECTION FLUID SPF 50 (OILY SKIN)
Mineral Sun protection fluid for oily and combination skin types. Enriched with Panthenol and Vitamin E. Suitable for face and body.
Brace Beauty, Embrace Purity.
www.celesty.com
Made in TURKEY
MABA KOZMETIK A.S.
ESENTEPE MAH. YUZBASI KAYA ALDOGAN SOK.
ONUR IS MERKEZI NO:12 D:8 ISTANBUL
Distributed by CELESTY LLC
3970 NW 167TH ST. MIAMI GARDENS,
FL 33054 - USA
info@celesty.com
- Packaging
-
INGREDIENTS AND APPEARANCE
CELESTY SUN PROTECTION SERIES SOLAR PROTECTION FLUID SPF 50
homosalate, avobenzone, octocrylene, octinoxate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81120-109 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 64 mg in 1 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 21.3 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 55.8 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 58.8 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CETYL ALCOHOL (UNII: 936JST6JCN) PEG-100 STEARATE (UNII: YD01N1999R) DIMETHICONE (UNII: 92RU3N3Y1O) PHENOXYETHANOL (UNII: HIE492ZZ3T) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ALLANTOIN (UNII: 344S277G0Z) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) TROLAMINE (UNII: 9O3K93S3TK) SODIUM POLYACRYLATE (2500000 MW) (UNII: 05I15JNI2J) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) PANTHENOL (UNII: WV9CM0O67Z) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) HYALURONATE SODIUM (UNII: YSE9PPT4TH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81120-109-01 1 in 1 BOX 12/07/2020 1 75 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/07/2020 Labeler - MABA KOZMETIK LIMITED SIRKETI (503001418) Establishment Name Address ID/FEI Business Operations MABA KOZMETIK LIMITED SIRKETI 503001418 manufacture(81120-109)