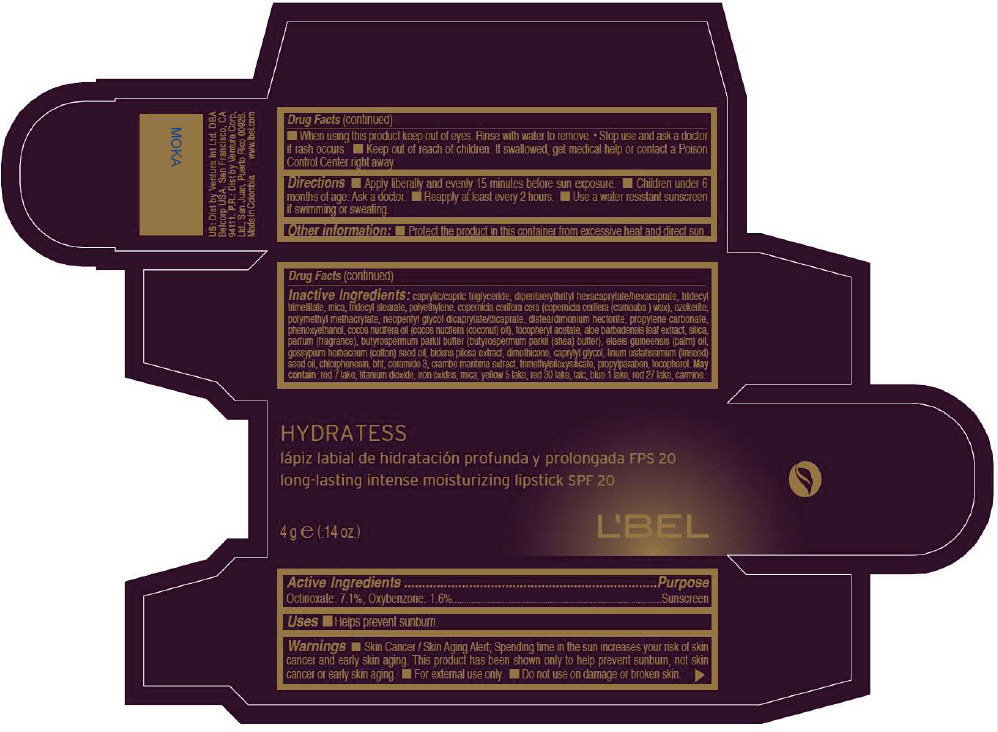
Label: LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - MOKA- octinoxate and oxybenzone lipstick
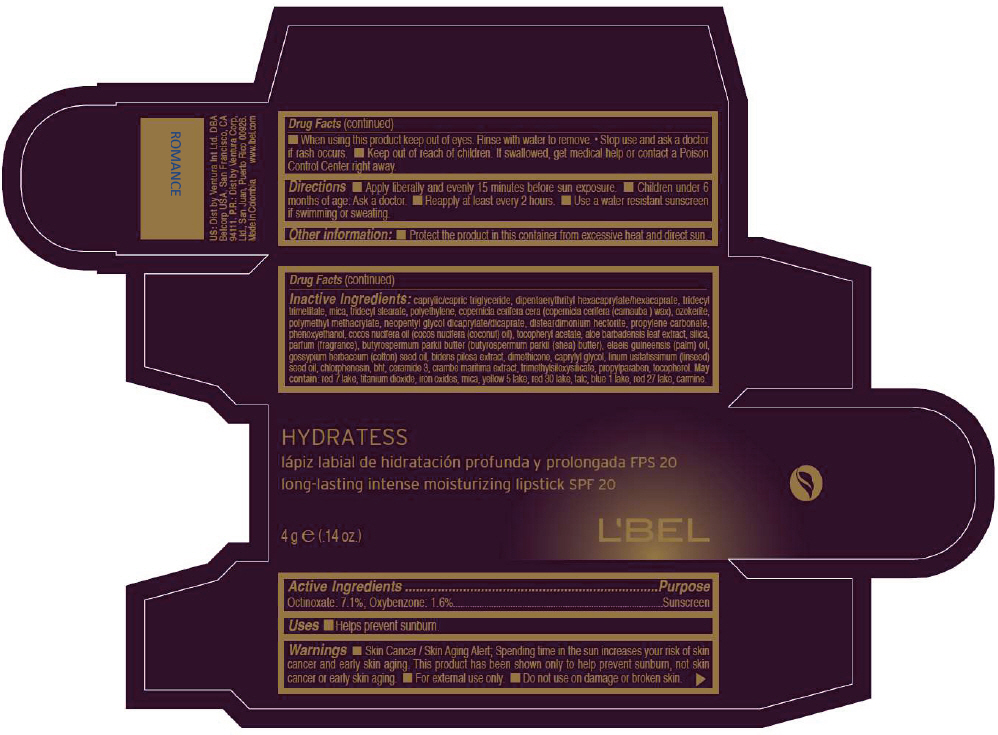
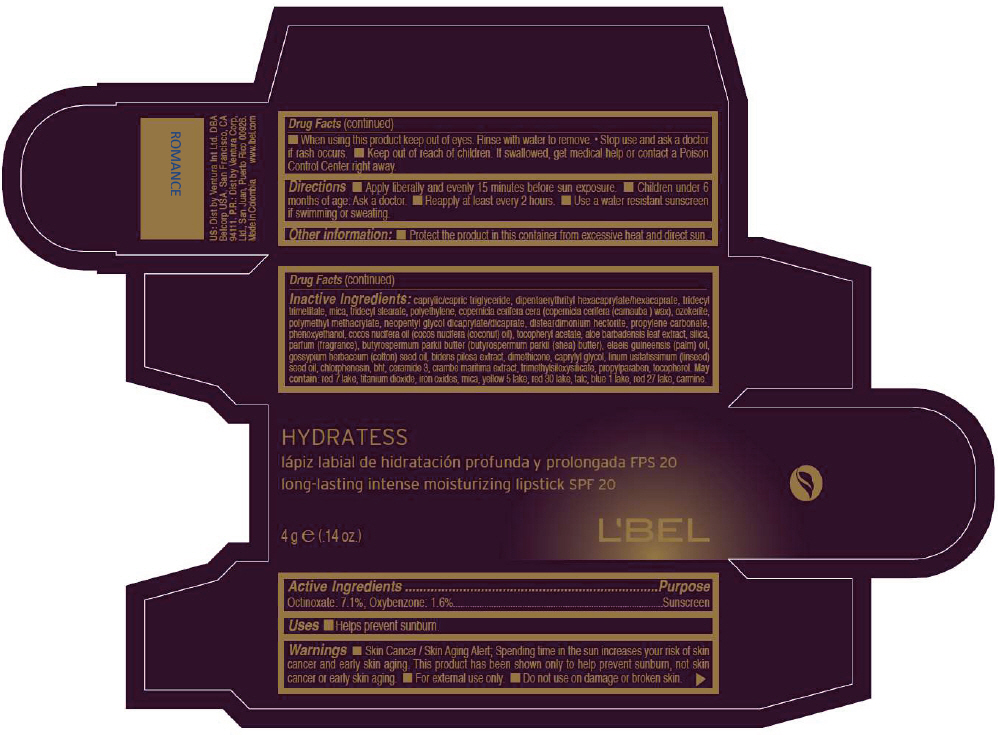
LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - ROMANCE- octinoxate and oxybenzone lipstick
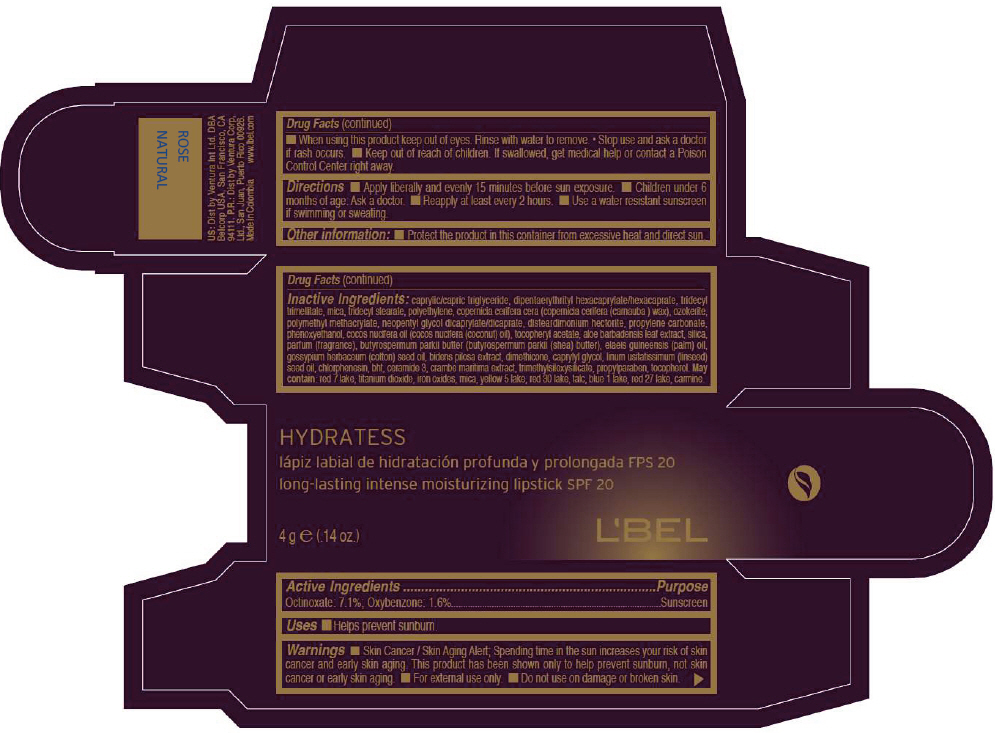
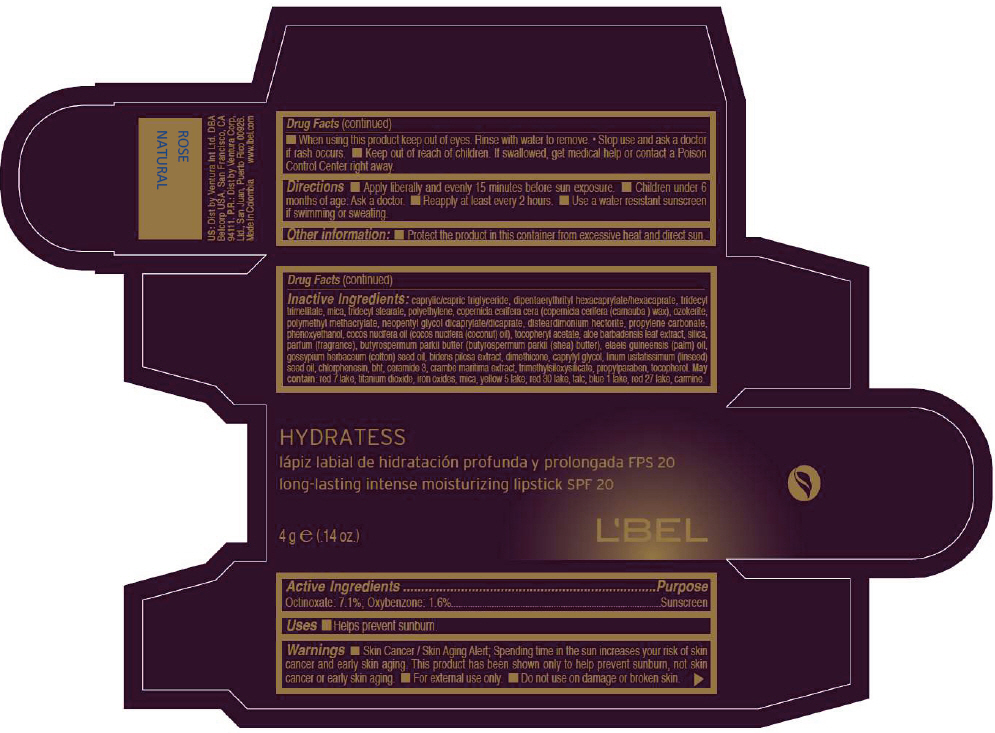
LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - ROSE NATURAL- octinoxate and oxybenzone lipstick
LBEL HYDRATESS LONG-LASTING INTENSE MOIST .......- NUDE- octinoxate and oxybenzone lipstick
LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - LILY BEIGE- octinoxate and oxybenzone lipstick
LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - MANDARINE- octinoxate and oxybenzone lipstick
LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - VIN- octinoxate and oxybenzone lipstick
-
Contains inactivated NDC Code(s)
NDC Code(s): 13537-511-01, 13537-511-02, 13537-512-01, 13537-512-02, view more13537-513-01, 13537-513-02, 13537-514-01, 13537-514-02, 13537-515-01, 13537-515-02, 13537-516-01, 13537-516-02, 13537-517-01, 13537-517-02, 13537-518-01, 13537-518-02, 13537-519-01, 13537-519-02, 13537-520-01, 13537-520-02, 13537-521-01, 13537-521-02, 13537-522-01, 13537-522-02, 13537-523-01, 13537-523-02, 13537-524-01, 13537-524-02, 13537-525-01, 13537-525-02 - Packager: Ventura Corporation LTD.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 6, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
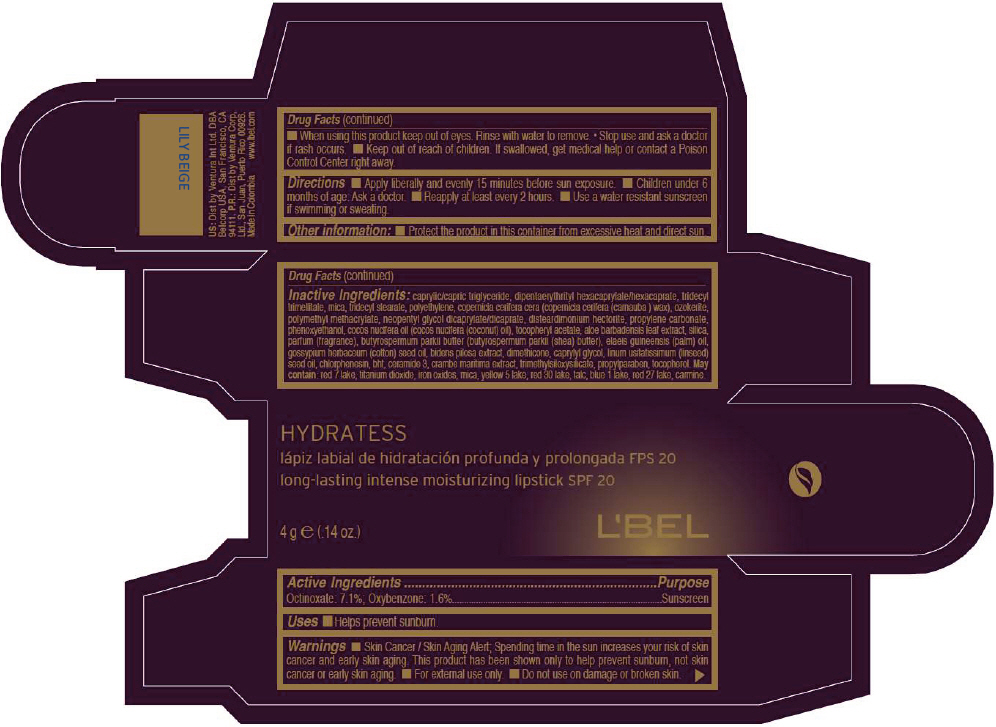
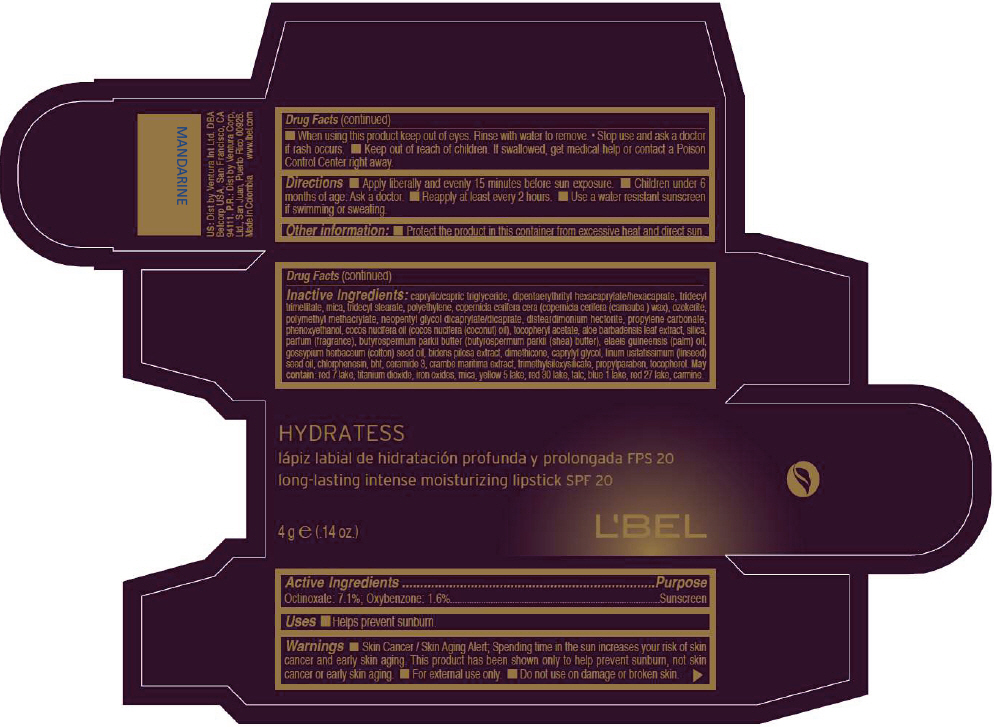
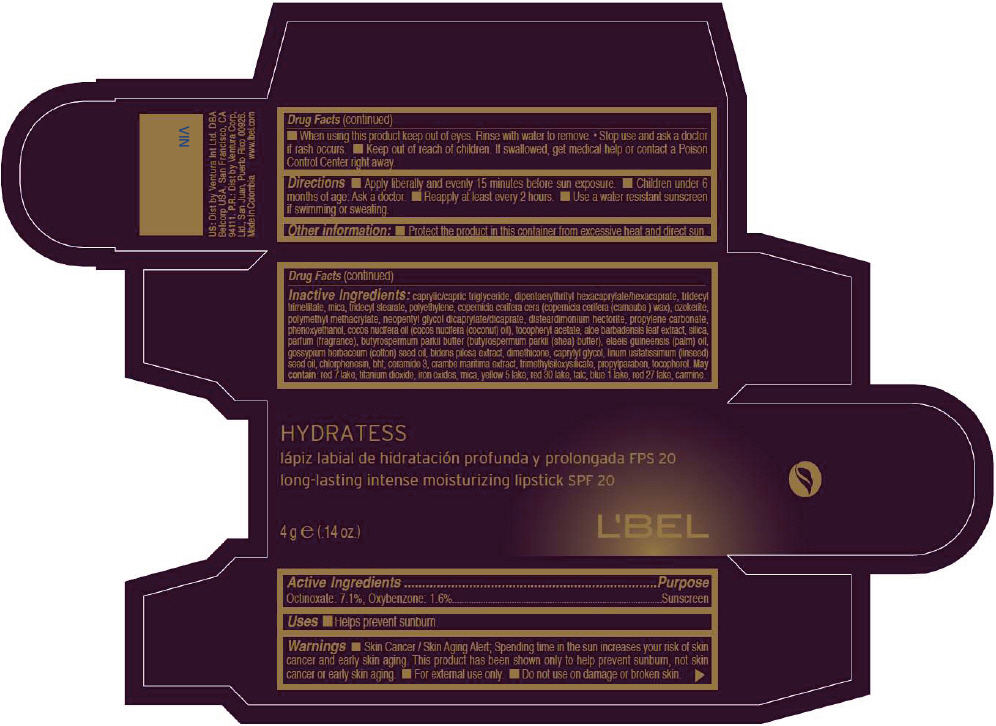
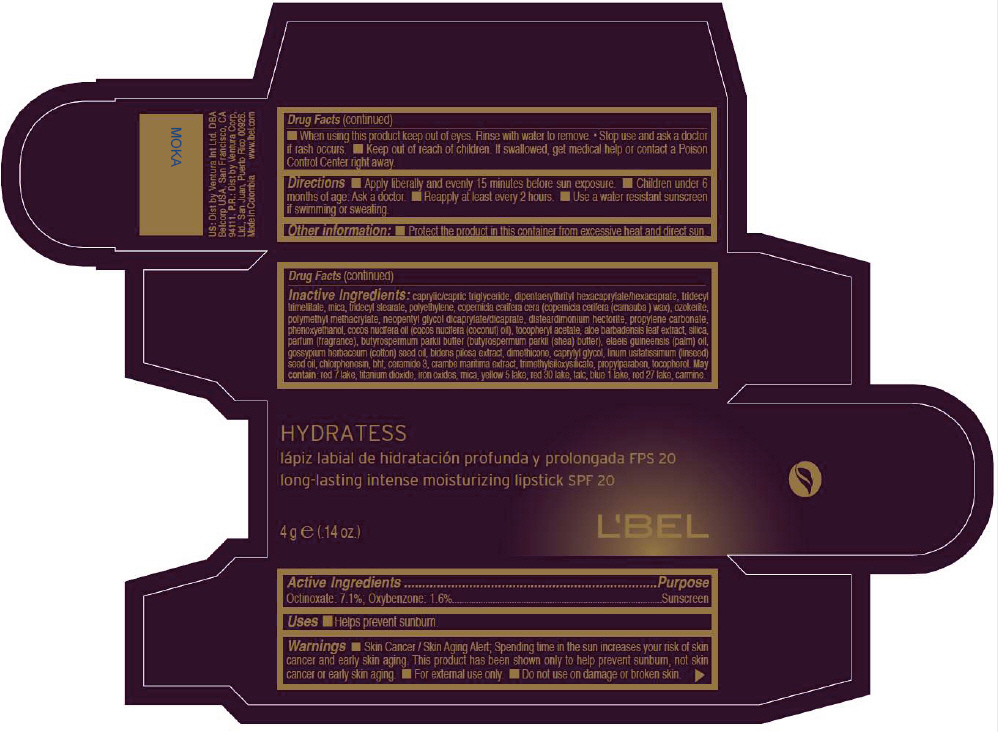
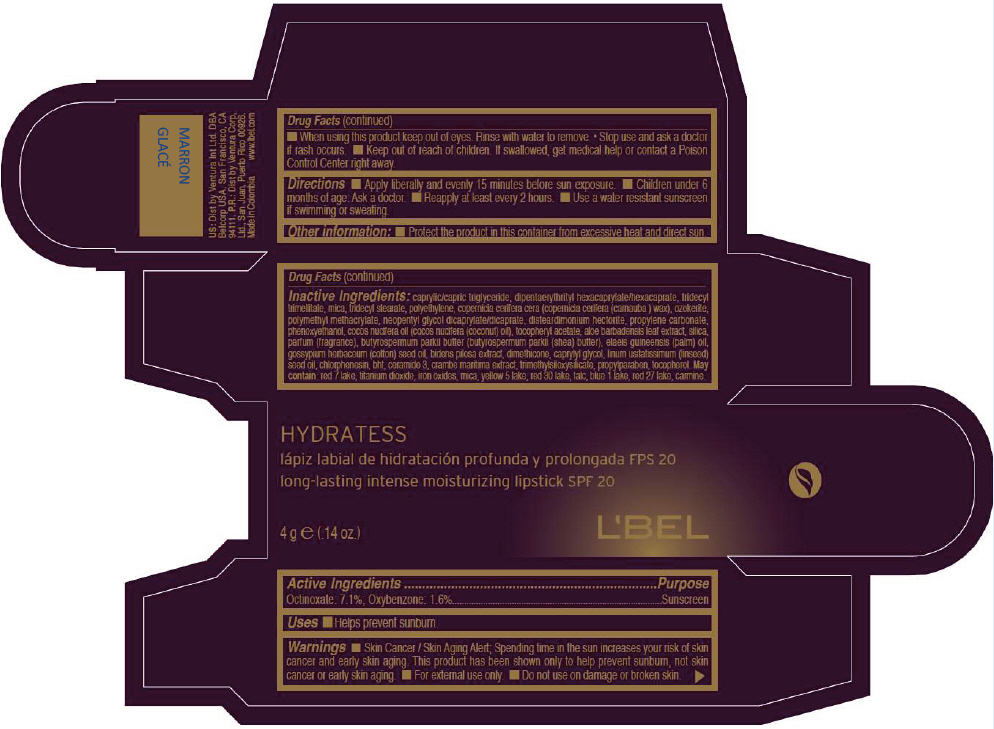
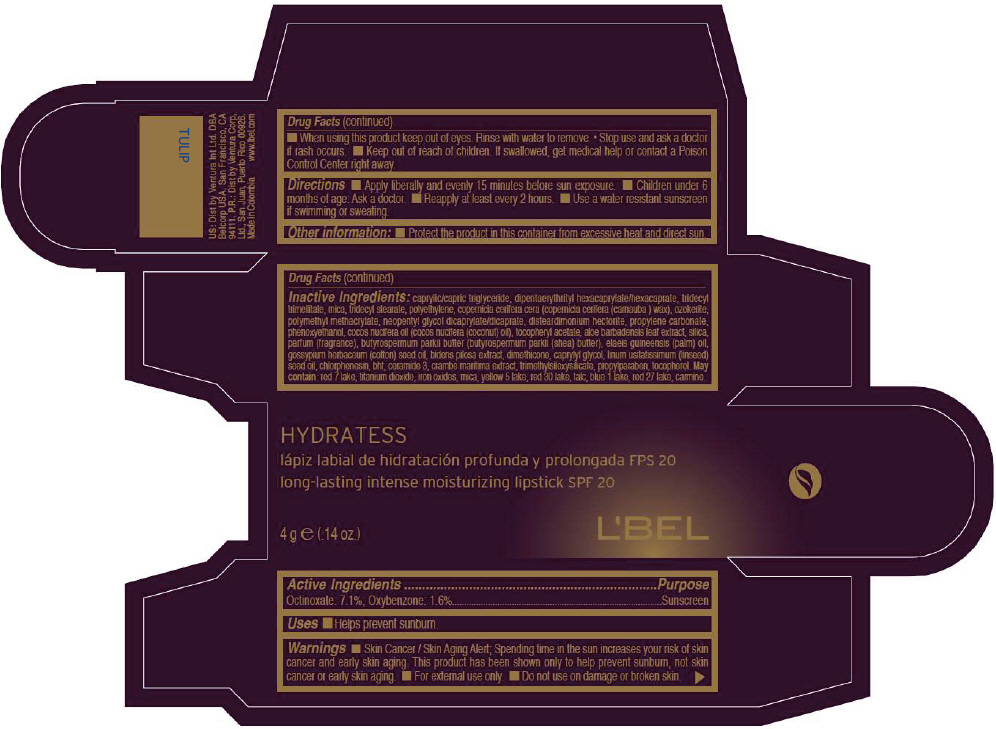
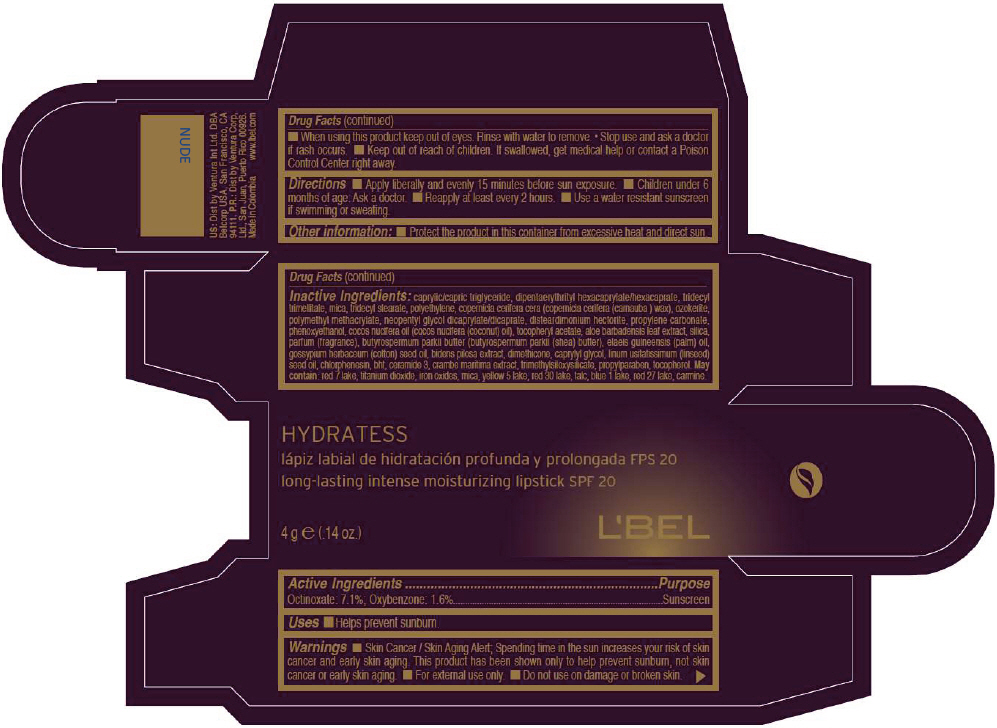
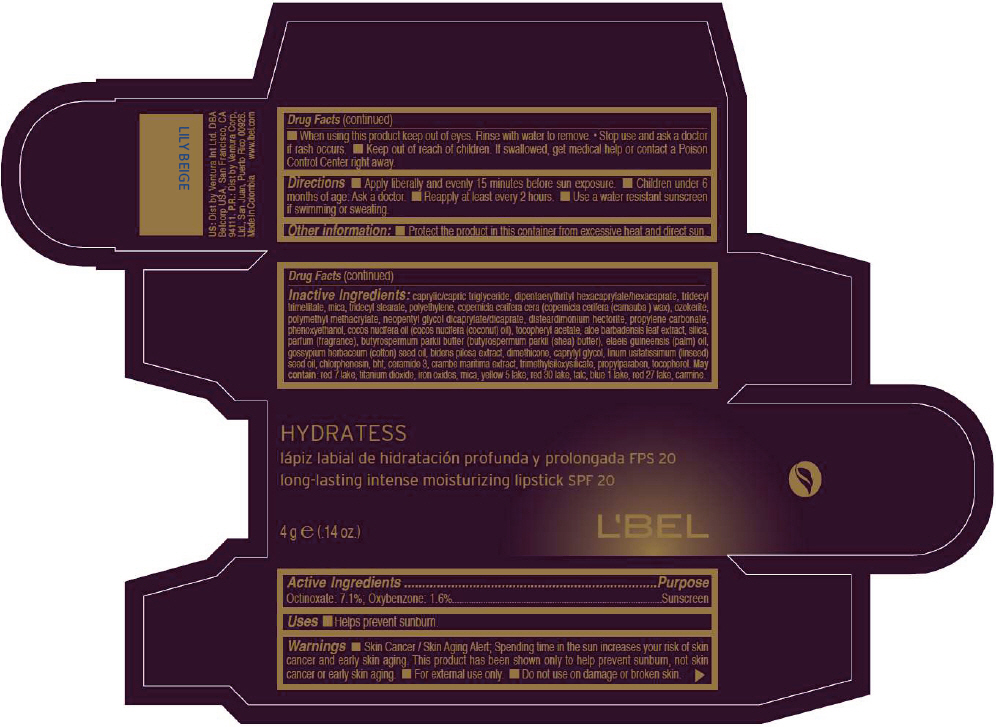
- Active Ingredients
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
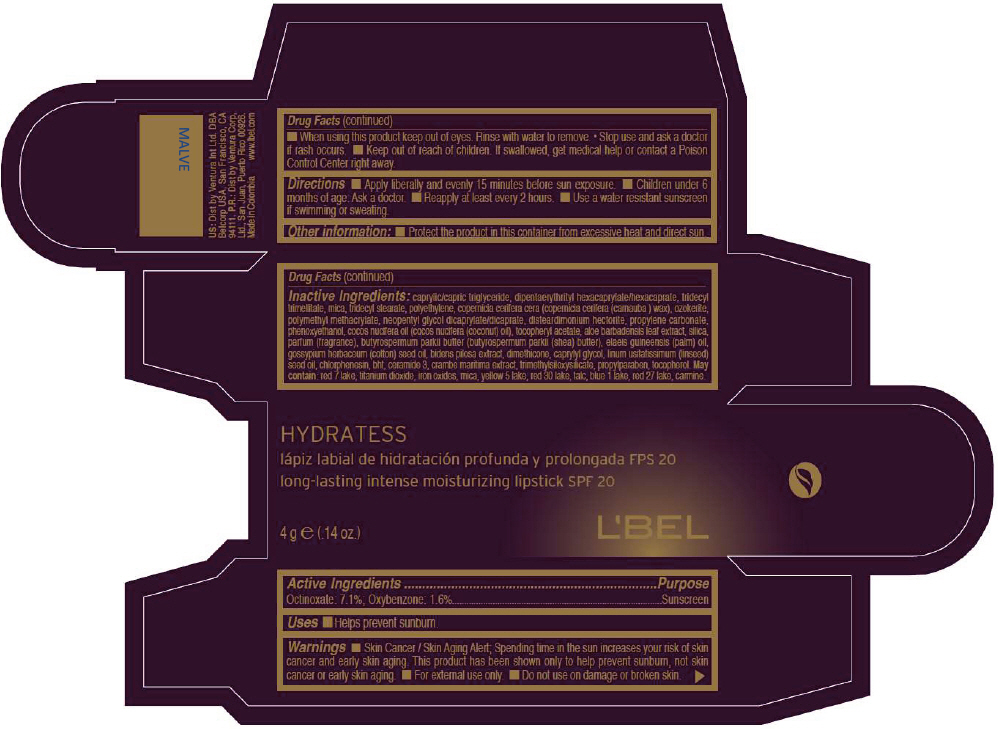
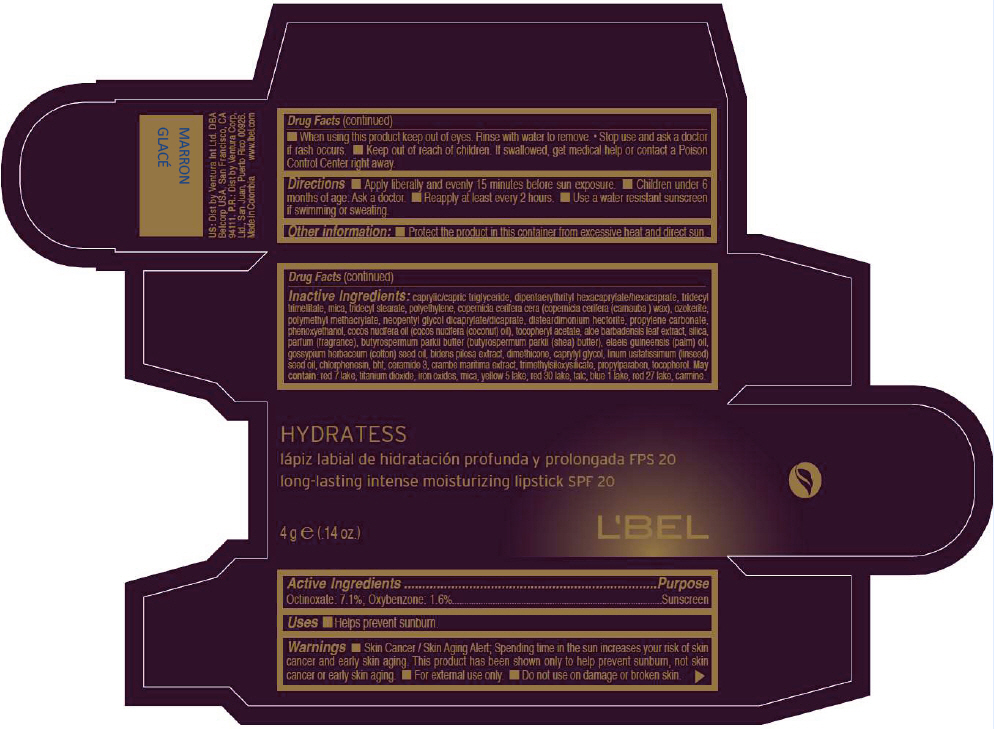
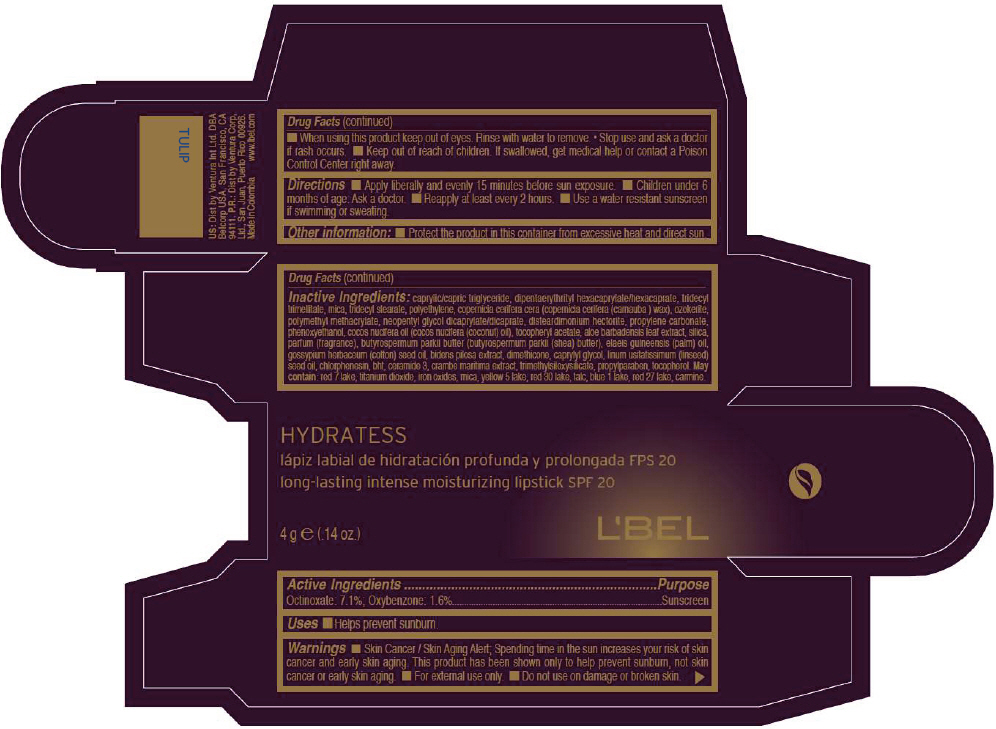
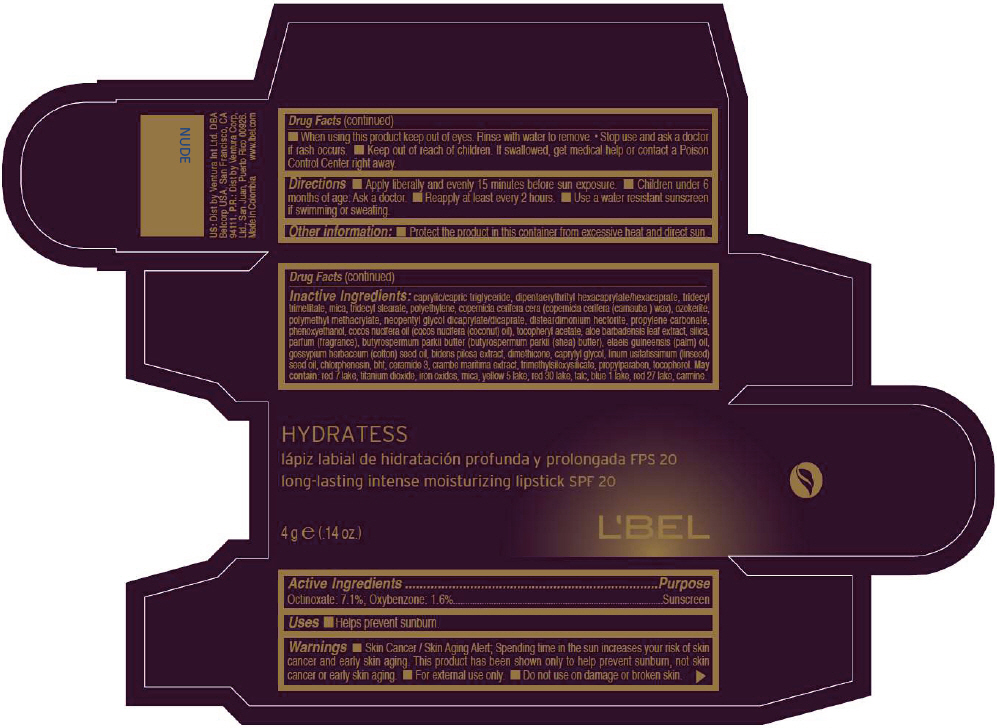
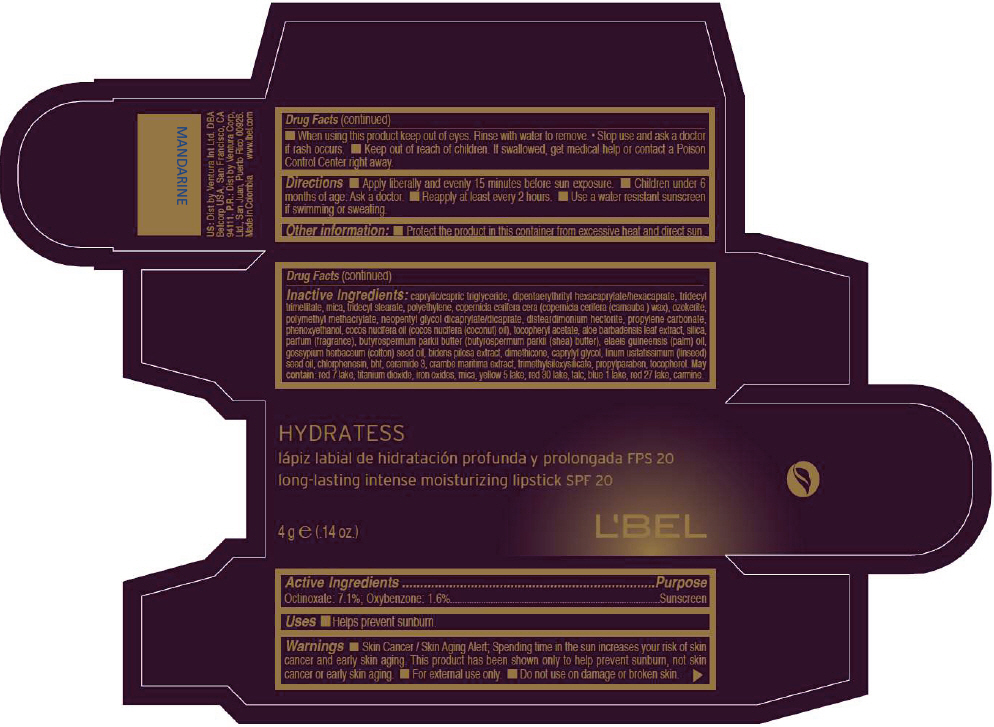
CAPRYLIC/CAPRIC TRIGLYCERIDE, DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE, TRIDECYL TRIMELLITATE, MICA, TRIDECYL STEARATE, POLYETHYLENE, COPERNICIA CERIFERA CERA (COPERNICIA CERIFERA (CARNAUBA ) WAX), OZOKERITE, POLYMETHYL METHACRYLATE, NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE, DISTEARDIMONIUM HECTORITE, PROPYLENE CARBONATE, PHENOXYETHANOL, COCOS NUCIFERA OIL (COCOS NUCIFERA (COCONUT) OIL), TOCOPHERYL ACETATE, ALOE BARBADENSIS LEAF EXTRACT, SILICA, PARFUM (FRAGRANCE), BUTYROSPERMUM PARKII BUTTER (BUTYROSPERMUM PARKII (SHEA) BUTTER), Elaeis Guineensis (Palm) Oil, Gossypium Herbaceum (Cotton) Seed Oil, Bidens Pilosa Extract, DIMETHICONE, CAPRYLYL GLYCOL, Linum Usitatissimum (Linseed) Seed Oil, CHLORPHENESIN, BHT, CERAMIDE 3, CRAMBE MARITIMA EXTRACT, TRIMETHYLSILOXYSILICATE, PROPYLPARABEN, TOCOPHEROL
MAY CONTAIN:
RED 7 LAKE, TITANIUM DIOXIDE, IRON OXIDES, MICA, YELLOW 5 LAKE, RED 30 LAKE, TALC, BLUE 1 LAKE, RED 27 LAKE, CARMINE. - SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - MOKA
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - ROMANCE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - ROSE NATURAL
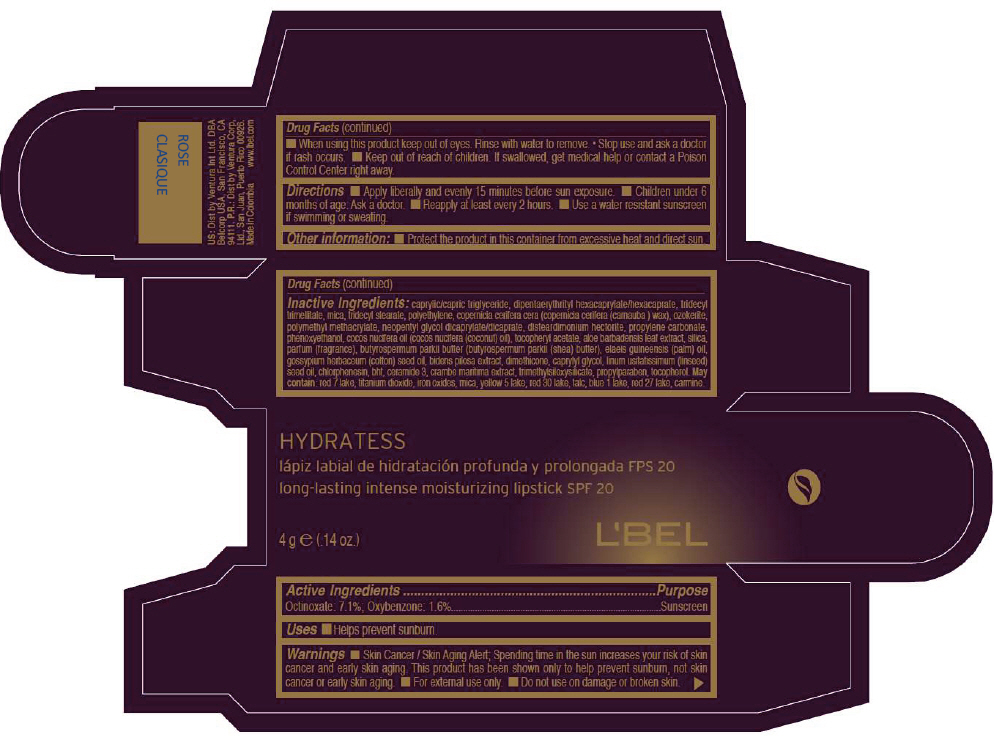
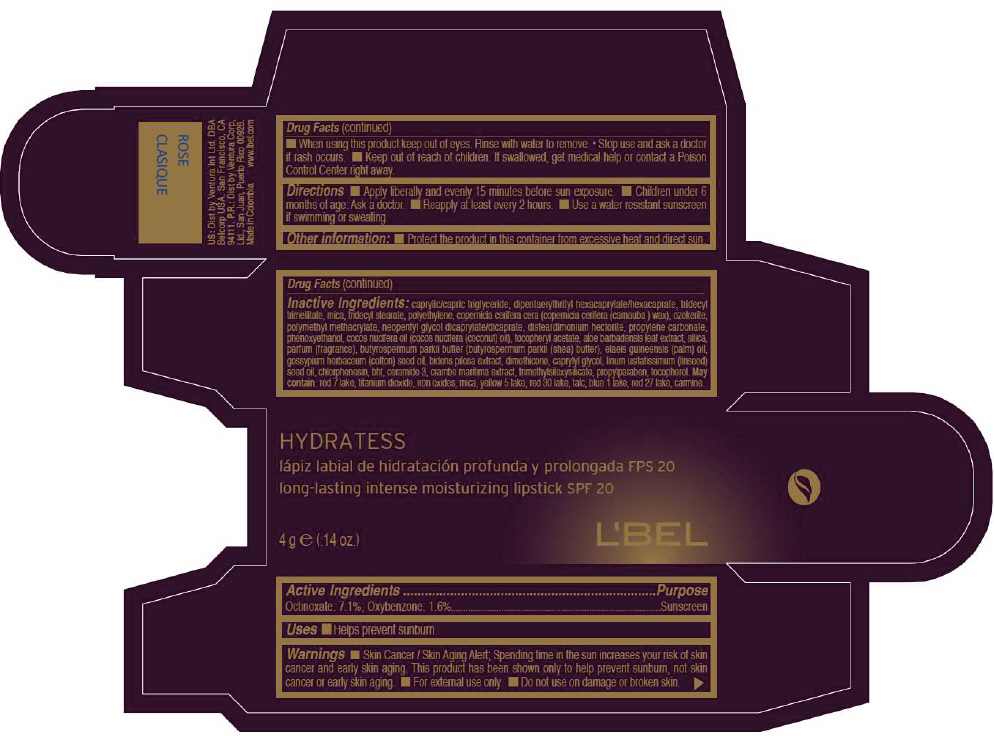
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - ROSE CLASIQUE
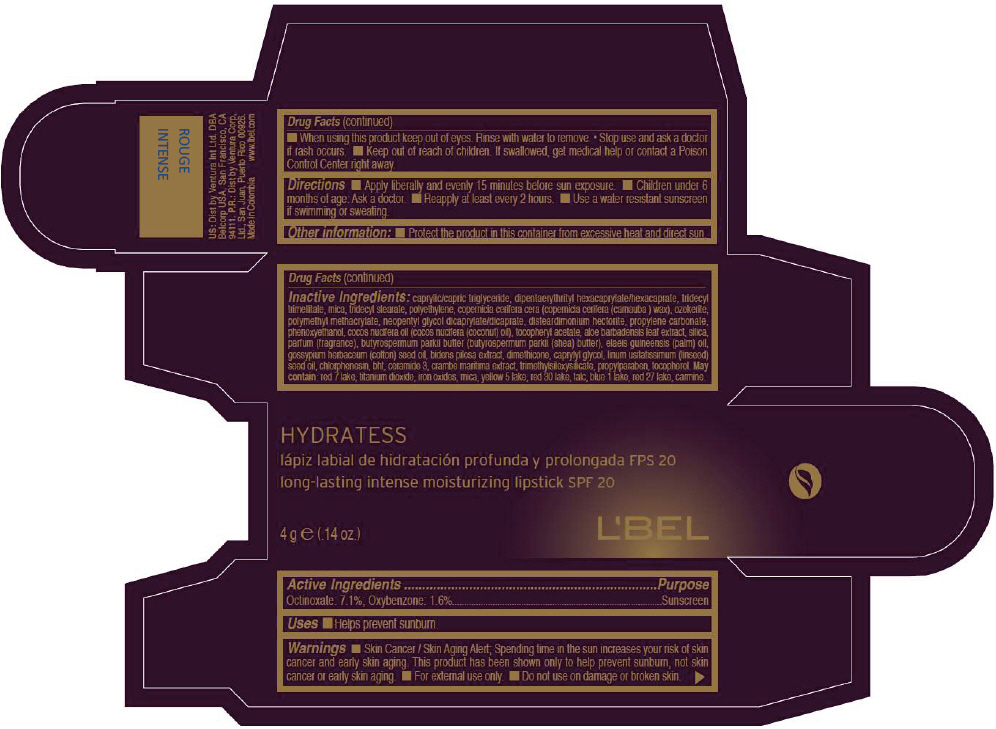
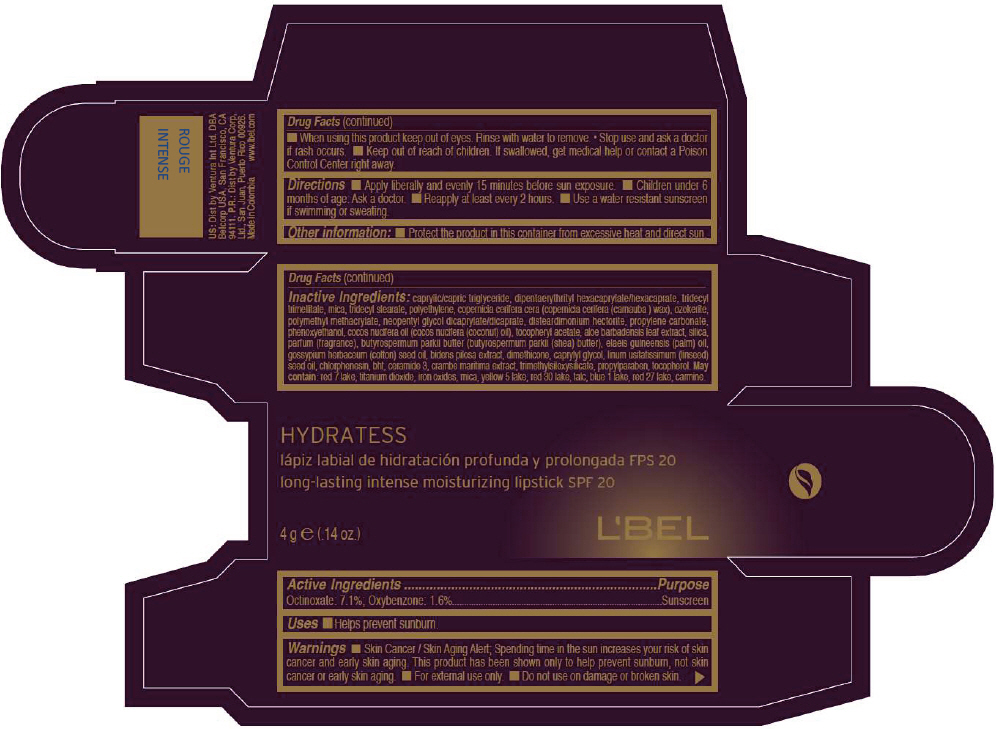
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - ROUGE INTENSE
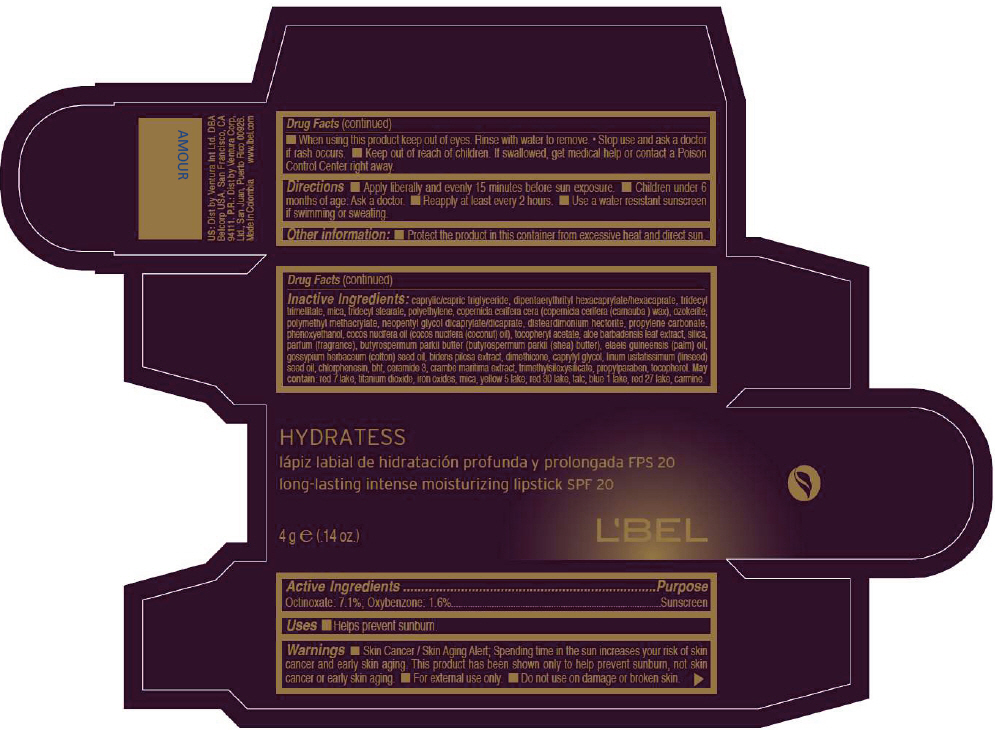
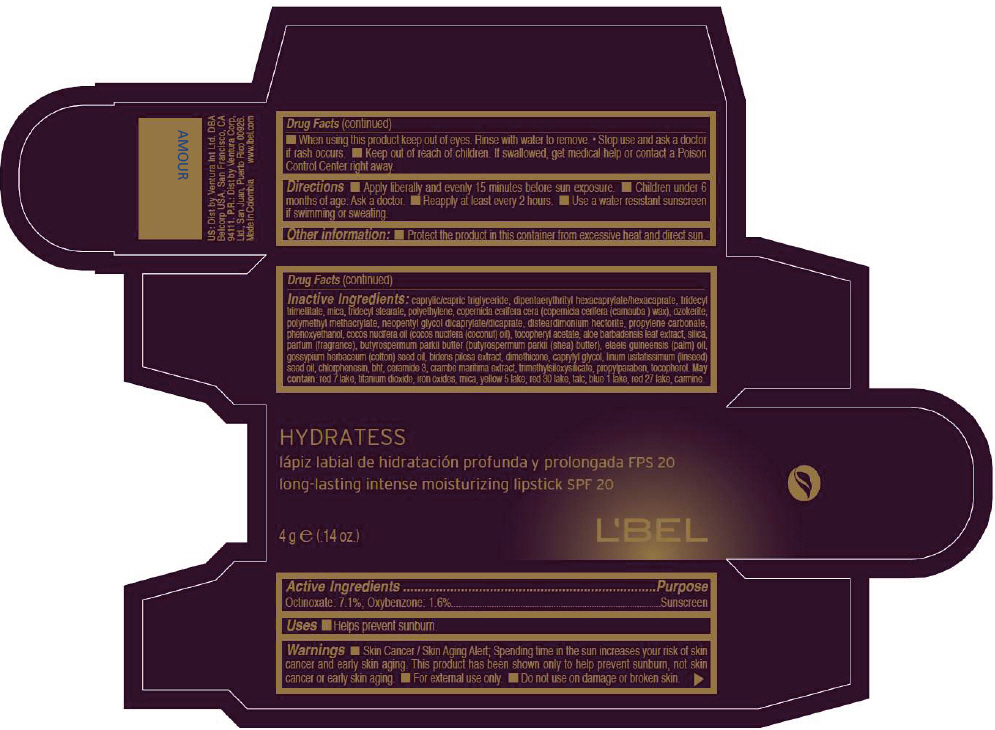
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - AMOUR
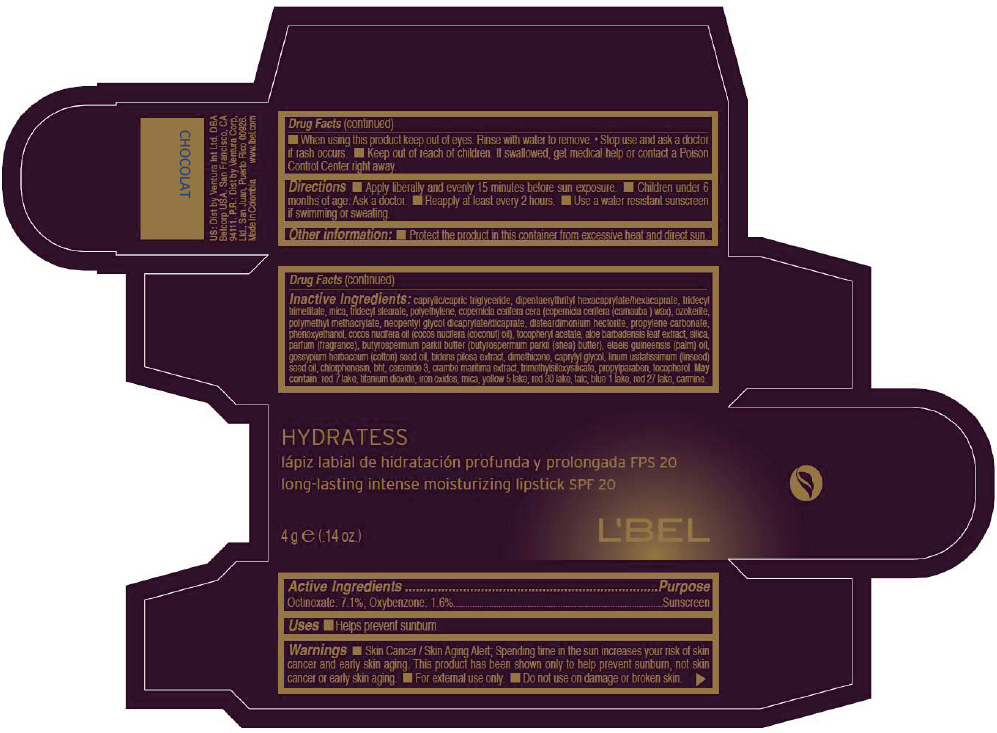
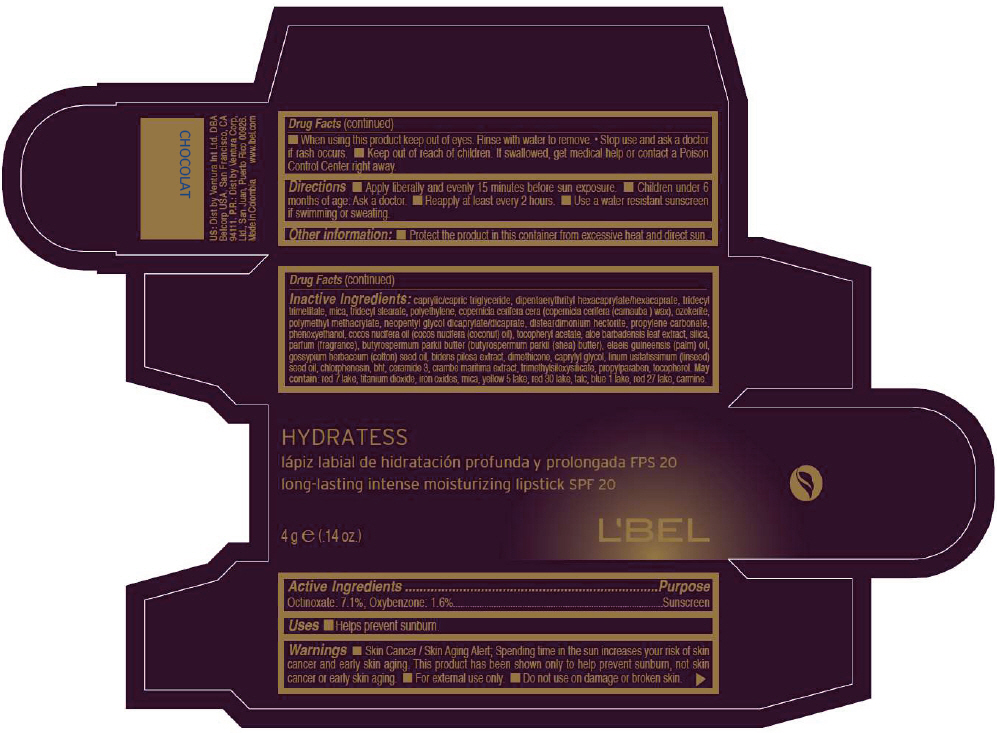
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - CHOCOLAT
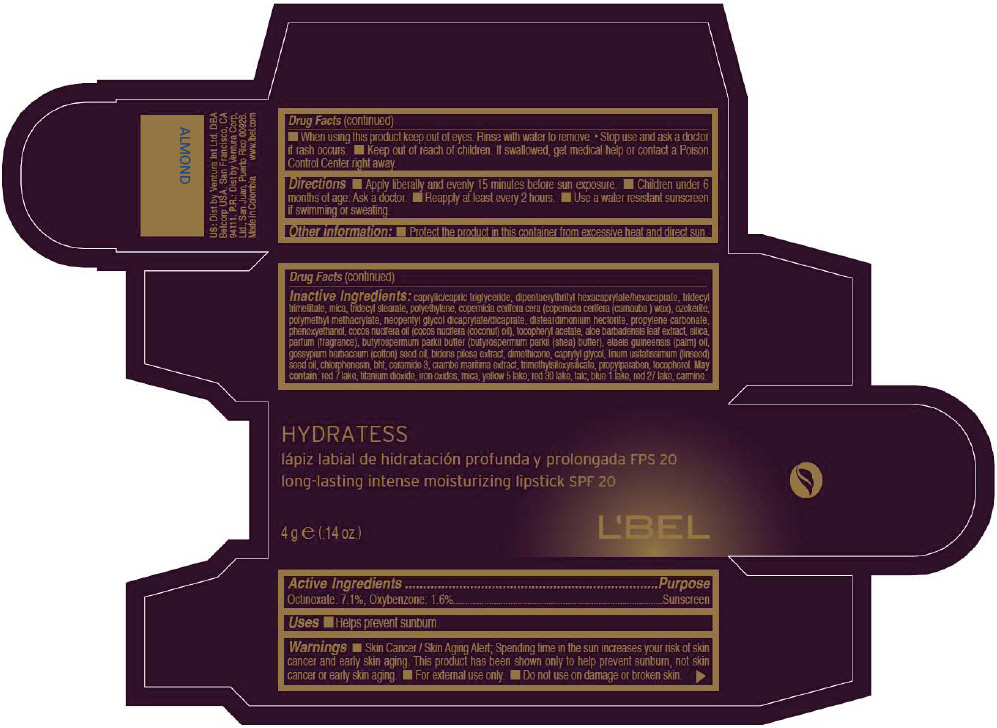
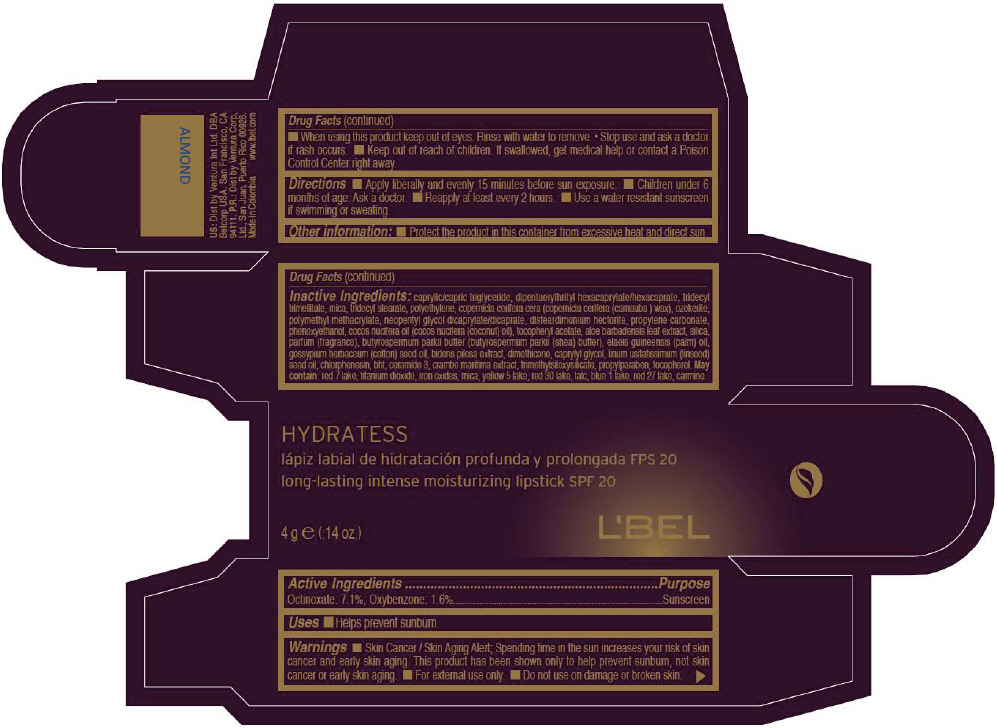
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - ALMOND
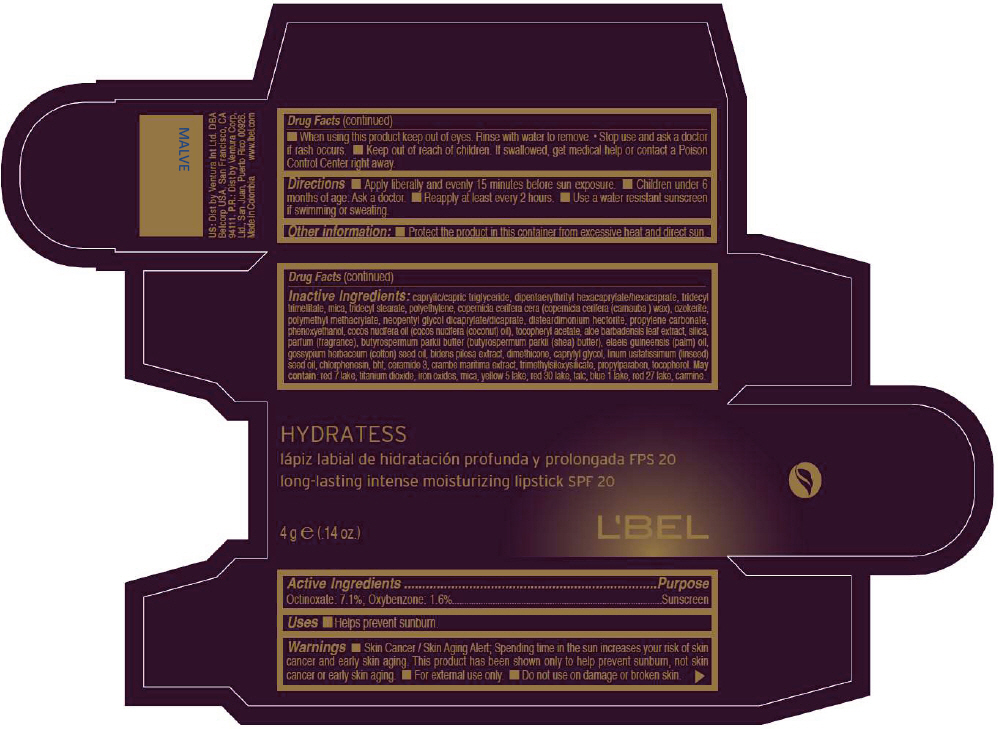
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - MALVE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - MARRON GLACÉ
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - TULIP
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - NUDE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - LILY BEIGE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - MANDARINE
- PRINCIPAL DISPLAY PANEL - 4 g Tube Box - VIN
-
INGREDIENTS AND APPEARANCE
LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - MOKA
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-511 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.071 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.016 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MICA (UNII: V8A1AW0880) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) Palm Oil (UNII: 5QUO05548Z) Levant Cottonseed Oil (UNII: N5CFT140R8) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Linseed Oil (UNII: 84XB4DV00W) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CERAMIDE 3 (UNII: 4370DF050B) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 30 (UNII: 2S42T2808B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color BROWN (Pink brown) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-511-02 1 in 1 BOX 1 NDC:13537-511-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2013 LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - ROMANCE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-512 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.071 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.016 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MICA (UNII: V8A1AW0880) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) Palm Oil (UNII: 5QUO05548Z) Levant Cottonseed Oil (UNII: N5CFT140R8) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Linseed Oil (UNII: 84XB4DV00W) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CERAMIDE 3 (UNII: 4370DF050B) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 30 (UNII: 2S42T2808B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color PINK (Dark magenta) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-512-02 1 in 1 BOX 1 NDC:13537-512-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2013 LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - ROSE NATURAL
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-513 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.071 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.016 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MICA (UNII: V8A1AW0880) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) Palm Oil (UNII: 5QUO05548Z) Levant Cottonseed Oil (UNII: N5CFT140R8) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Linseed Oil (UNII: 84XB4DV00W) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CERAMIDE 3 (UNII: 4370DF050B) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 30 (UNII: 2S42T2808B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color PINK (Intense yellowish pink) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-513-02 1 in 1 BOX 1 NDC:13537-513-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2013 LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - ROSE CLASIQUE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-514 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.071 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.016 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MICA (UNII: V8A1AW0880) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) Palm Oil (UNII: 5QUO05548Z) Levant Cottonseed Oil (UNII: N5CFT140R8) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Linseed Oil (UNII: 84XB4DV00W) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CERAMIDE 3 (UNII: 4370DF050B) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 30 (UNII: 2S42T2808B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color PINK (Intense bluish pink) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-514-02 1 in 1 BOX 1 NDC:13537-514-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2013 LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - ROUGE INTENSE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-515 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.071 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.016 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MICA (UNII: V8A1AW0880) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) Palm Oil (UNII: 5QUO05548Z) Levant Cottonseed Oil (UNII: N5CFT140R8) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Linseed Oil (UNII: 84XB4DV00W) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CERAMIDE 3 (UNII: 4370DF050B) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 30 (UNII: 2S42T2808B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color RED (Bluish red) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-515-02 1 in 1 BOX 1 NDC:13537-515-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2013 LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - AMOUR
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-516 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.071 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.016 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MICA (UNII: V8A1AW0880) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) Palm Oil (UNII: 5QUO05548Z) Levant Cottonseed Oil (UNII: N5CFT140R8) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Linseed Oil (UNII: 84XB4DV00W) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CERAMIDE 3 (UNII: 4370DF050B) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 30 (UNII: 2S42T2808B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color BROWN (Red brown) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-516-02 1 in 1 BOX 1 NDC:13537-516-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2013 LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - CHOCOLAT
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-517 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.071 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.016 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MICA (UNII: V8A1AW0880) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) Palm Oil (UNII: 5QUO05548Z) Levant Cottonseed Oil (UNII: N5CFT140R8) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Linseed Oil (UNII: 84XB4DV00W) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CERAMIDE 3 (UNII: 4370DF050B) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 30 (UNII: 2S42T2808B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color BROWN (Yellowish brown) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-517-02 1 in 1 BOX 1 NDC:13537-517-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2013 LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - ALMOND
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-518 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.071 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.016 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MICA (UNII: V8A1AW0880) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) Palm Oil (UNII: 5QUO05548Z) Levant Cottonseed Oil (UNII: N5CFT140R8) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Linseed Oil (UNII: 84XB4DV00W) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CERAMIDE 3 (UNII: 4370DF050B) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 30 (UNII: 2S42T2808B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color BROWN (Reddish brown) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-518-02 1 in 1 BOX 1 NDC:13537-518-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2013 LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - MALVE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-519 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.071 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.016 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MICA (UNII: V8A1AW0880) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) Palm Oil (UNII: 5QUO05548Z) Levant Cottonseed Oil (UNII: N5CFT140R8) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Linseed Oil (UNII: 84XB4DV00W) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CERAMIDE 3 (UNII: 4370DF050B) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 30 (UNII: 2S42T2808B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color RED (Yellowish red) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-519-02 1 in 1 BOX 1 NDC:13537-519-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2013 LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - MARRON GLACE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-520 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.071 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.016 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MICA (UNII: V8A1AW0880) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) Palm Oil (UNII: 5QUO05548Z) Levant Cottonseed Oil (UNII: N5CFT140R8) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Linseed Oil (UNII: 84XB4DV00W) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CERAMIDE 3 (UNII: 4370DF050B) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 30 (UNII: 2S42T2808B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color BROWN (Purplish brown) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-520-02 1 in 1 BOX 1 NDC:13537-520-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2013 LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - TULIP
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-521 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.071 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.016 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MICA (UNII: V8A1AW0880) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) Palm Oil (UNII: 5QUO05548Z) Levant Cottonseed Oil (UNII: N5CFT140R8) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Linseed Oil (UNII: 84XB4DV00W) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CERAMIDE 3 (UNII: 4370DF050B) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 30 (UNII: 2S42T2808B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color PINK (Yellowish pink) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-521-02 1 in 1 BOX 1 NDC:13537-521-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2013 LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - NUDE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-522 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.071 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.016 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MICA (UNII: V8A1AW0880) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) Palm Oil (UNII: 5QUO05548Z) Levant Cottonseed Oil (UNII: N5CFT140R8) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Linseed Oil (UNII: 84XB4DV00W) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CERAMIDE 3 (UNII: 4370DF050B) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 30 (UNII: 2S42T2808B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color PINK (Light Yellowish pink) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-522-02 1 in 1 BOX 1 NDC:13537-522-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2013 LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - LILY BEIGE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-523 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.071 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.016 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MICA (UNII: V8A1AW0880) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) Palm Oil (UNII: 5QUO05548Z) Levant Cottonseed Oil (UNII: N5CFT140R8) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Linseed Oil (UNII: 84XB4DV00W) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CERAMIDE 3 (UNII: 4370DF050B) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 30 (UNII: 2S42T2808B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color BROWN (Light pinkish brown) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-523-02 1 in 1 BOX 1 NDC:13537-523-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2013 LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - MANDARINE
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-524 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.071 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.016 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MICA (UNII: V8A1AW0880) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) Palm Oil (UNII: 5QUO05548Z) Levant Cottonseed Oil (UNII: N5CFT140R8) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Linseed Oil (UNII: 84XB4DV00W) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CERAMIDE 3 (UNII: 4370DF050B) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 30 (UNII: 2S42T2808B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color ORANGE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-524-02 1 in 1 BOX 1 NDC:13537-524-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2013 LBEL HYDRATESS LONG-LASTING INTENSE MOISTURIZING SPF 20 - VIN
octinoxate and oxybenzone lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13537-525 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 0.071 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.016 g in 1 g Inactive Ingredients Ingredient Name Strength MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) DIPENTAERYTHRITYL HEXACAPRYLATE/HEXACAPRATE (UNII: 554N82UWVW) TRIDECYL TRIMELLITATE (UNII: FY36J270ES) MICA (UNII: V8A1AW0880) TRIDECYL STEARATE (UNII: A8OE252M6L) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) CARNAUBA WAX (UNII: R12CBM0EIZ) NEOPENTYL GLYCOL DICAPRYLATE/DICAPRATE (UNII: VLW429K27K) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE CARBONATE (UNII: 8D08K3S51E) PHENOXYETHANOL (UNII: HIE492ZZ3T) COCONUT OIL (UNII: Q9L0O73W7L) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ALOE VERA LEAF (UNII: ZY81Z83H0X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHEA BUTTER (UNII: K49155WL9Y) Palm Oil (UNII: 5QUO05548Z) Levant Cottonseed Oil (UNII: N5CFT140R8) DIMETHICONE (UNII: 92RU3N3Y1O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Linseed Oil (UNII: 84XB4DV00W) CHLORPHENESIN (UNII: I670DAL4SZ) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CERAMIDE 3 (UNII: 4370DF050B) PROPYLPARABEN (UNII: Z8IX2SC1OH) TOCOPHEROL (UNII: R0ZB2556P8) D&C RED NO. 7 (UNII: ECW0LZ41X8) ALUMINUM OXIDE (UNII: LMI26O6933) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 30 (UNII: 2S42T2808B) TALC (UNII: 7SEV7J4R1U) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C RED NO. 27 (UNII: 2LRS185U6K) Product Characteristics Color PURPLE (Brownish purple) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13537-525-02 1 in 1 BOX 1 NDC:13537-525-01 4 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 05/27/2013 Labeler - Ventura Corporation LTD. (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(13537-511, 13537-512, 13537-513, 13537-514, 13537-515, 13537-516, 13537-517, 13537-518, 13537-519, 13537-520, 13537-521, 13537-522, 13537-523, 13537-524, 13537-525)