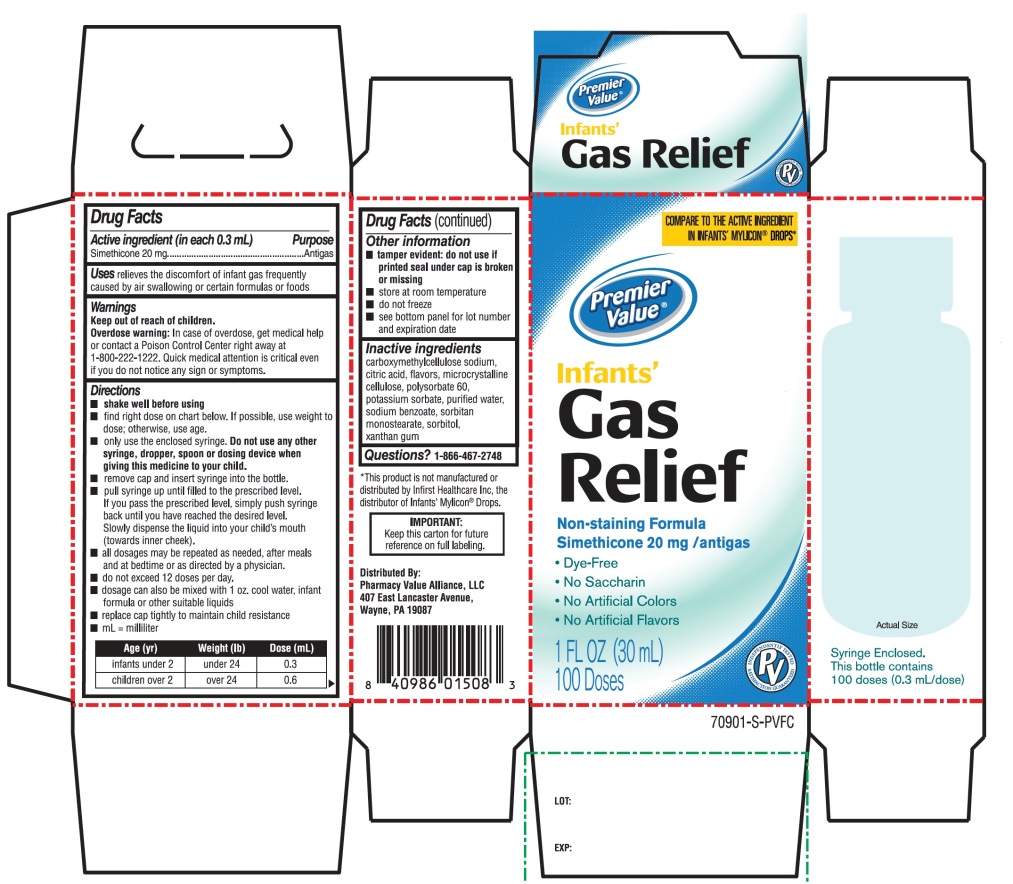
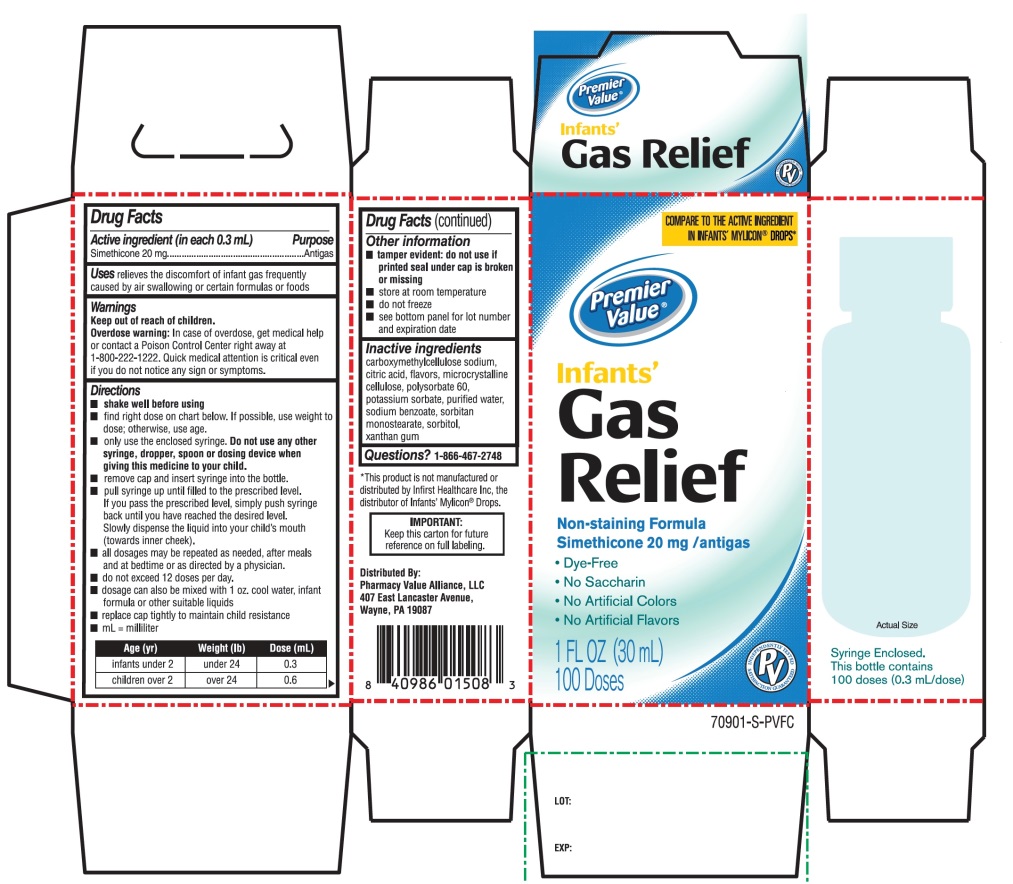
Label: PREMIER VALUE INFANTS GAS RELIEF- simethicone emulsion
- NDC Code(s): 68016-670-00
- Packager: Chain Drug Consortium
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each 0.3 mL)
- Purpose
- Uses
- Warnings
-
Directions
- ▪
- shake well before using
- ▪
- all dosages may be repeated as needed, after meals and at bedtime or as directed by a physician. Do not exceed 12 doses per day.
- ▪
- fill enclosed dropper to recommended dosage level and dispense liquid slowly into baby’s mouth, toward the inner cheek
- ▪
- dosage can also be mixed with 1 oz. cool water, infant formula or other suitable liquids
- ▪
- clean dropper after each use - replace bottle with original cap
age (yr)
weight (lb)
dose
infants under 2
under 24
0.3 mL
children over 2
over 24
0.6 mL
- Other information
- Inactive ingredients
-
Principal Display Panel
COMPARE TO ACTIVE INGREDIENT IN INFANTS' MYLICON® DROPS*
Premier Value®
Infants'
Gas Relief
Non-Staining formula
Simethicone 20 mg/Antigas
- •
- Dye Free
- •
- No Saccharin
- •
- No Artificial Colors
- •
- No Artificial flavors
Syringe Enclosed. This Bottle contains 100 doses (0.3 mL/dose)
1 Fl. OZ (30ml)
100 Doses
Distributed by
Pharmacy Value Alliance, LLC
407 East Lancaster Avenue,
Wayne, PA 19087
*This product is not manufactured or distributed by Infirst Healthcare Inc., the distributor of Infants’ Mylicon® Drops.
-
INGREDIENTS AND APPEARANCE
PREMIER VALUE INFANTS GAS RELIEF
simethicone emulsionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-670 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 20 mg in 0.3 mL Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYSORBATE 60 (UNII: CAL22UVI4M) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) SORBITOL (UNII: 506T60A25R) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color WHITE (white to off white, opaque) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-670-00 1 in 1 CARTON 01/09/2015 1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M002 01/09/2015 Labeler - Chain Drug Consortium (101668460)