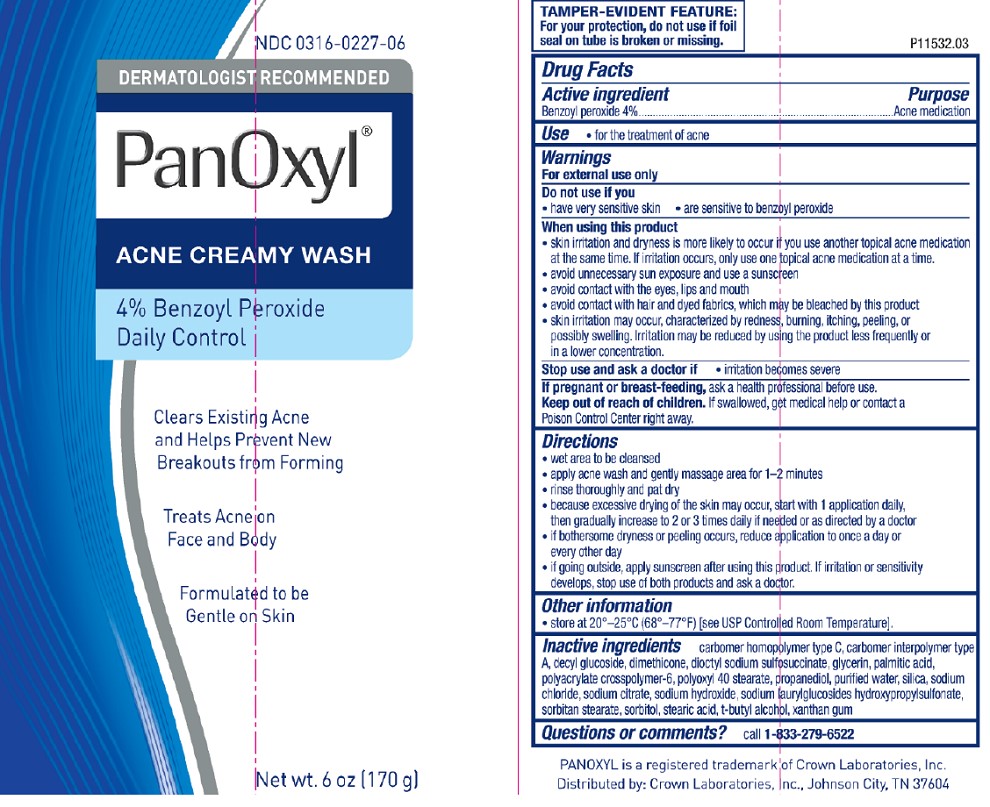
Label: PANOXYL- benzoyl peroxide cream
- NDC Code(s): 0316-0227-06
- Packager: Crown Laboratories
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated April 18, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
When using this product
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time
- avoid unnecessary sun exposure and use a sunscreen
- avoid contact with the eyes, lips and mouth
- avoid contact with hair and dyed fabrics, which may be bleached by this product
- skin irritation may occur, characterized by redness, burning, itching, peeling, or possibly swelling. Irritation may be reduced by using the product less frequently or in a lower concentration.
-
Directions
- wet area to be cleansed
- apply acne wash and gently massage area for 1-2 minutes
- rinse thoroughly and pat dry
- because excessive drying of the skin may occur, start with 1 application daily, then gradually increase to 2 or 3 times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day
- if going outside, apply sunscreen after using this product. If irritation or sensitivity develops, stop use of both products and ask a doctor.
- Other information
-
Inactive ingredients (4%)
carbomer homopolymer type C, carbomer interpolymer type A, decyl glucoside, dimethicone, dioctyl sodium sulfosuccinate, glycerin, palmitic acid, polyacrylate crosspolymer-6, polyoxyl 40 stearate, propanediol, purified water, silica, sodium chloride, sodium citrate, sodium hydroxide, sodium laurylglucosides hydroxypropylsulfonate, sorbitan stearate, sorbitol, stearic acid, t-butyl alcohol, xanthan gum
- Questions or comments?
-
Panoxyl 4% Tube
NDC 0316-0227-06
DERMATOLOGIST RECOMMENDED
PanOxyl®
ACNE CREAMY WASH
4% Benzoyl Peroxide
Daily Control
Clears Existing Acne and Helps Prevent New Breakouts from Forming
Treats Acne on Face and Body
Formulated to be Gentle on Skin
Net wt. 6oz (170g)
TAMPER-EVIDENT FEATURE:
For your protection, do not use if foil seal on tube is broken or missing.
P11532.03
PANOXYL is a registered trademark of Crown Laboratories, Inc.
Distributed by: Crown Laboratories, Inc. Johnson City, TN 37604
-
Panoxyl 4% Carton
NDC 0316-0227-06
DERMATOLOGIST RECOMMENDED
PanOxyl®
ACNE CREAMY WASH
4% Benzoyl Peroxide
Daily Control
Clears Existing Acne and Helps Prevent New Breakouts from Forming
Treats Acne on Face and Body
Formulated to be Gentle on Skin
Net wt. 6oz (170g)
PANOXYL is a registered trademark of Crown Laboratories, Inc.
Distributed by: Crown Laboratories, Inc. Johnson City, TN 37604
P11730.02

-
INGREDIENTS AND APPEARANCE
PANOXYL
benzoyl peroxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0316-0227 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOYL PEROXIDE (UNII: W9WZN9A0GM) (BENZOYL PEROXIDE - UNII:W9WZN9A0GM) BENZOYL PEROXIDE 58.7 mg in 1 g Inactive Ingredients Ingredient Name Strength XANTHAN GUM (UNII: TTV12P4NEE) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) DIMETHICONE (UNII: 92RU3N3Y1O) DOCUSATE SODIUM (UNII: F05Q2T2JA0) PALMITIC ACID (UNII: 2V16EO95H1) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM CHLORIDE (UNII: 451W47IQ8X) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) WATER (UNII: 059QF0KO0R) AMMONIUM ACRYLOYLDIMETHYLTAURATE, DIMETHYLACRYLAMIDE, LAURYL METHACRYLATE AND LAURETH-4 METHACRYLATE COPOLYMER, TRIMETHYLOLPROPANE TRIACRYLATE CROSSLINKED (45000 MPA.S) (UNII: Q7UI015FF9) SORBITOL (UNII: 506T60A25R) GLYCERIN (UNII: PDC6A3C0OX) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) STEARIC ACID (UNII: 4ELV7Z65AP) CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) SODIUM HYDROXIDE (UNII: 55X04QC32I) POLYOXYL 40 STEARATE (UNII: 13A4J4NH9I) PROPANEDIOL (UNII: 5965N8W85T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0316-0227-06 1 in 1 CARTON 12/01/2018 1 170 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 03/25/2011 Labeler - Crown Laboratories (079035945) Establishment Name Address ID/FEI Business Operations Crown Laboratories 079035945 manufacture(0316-0227)