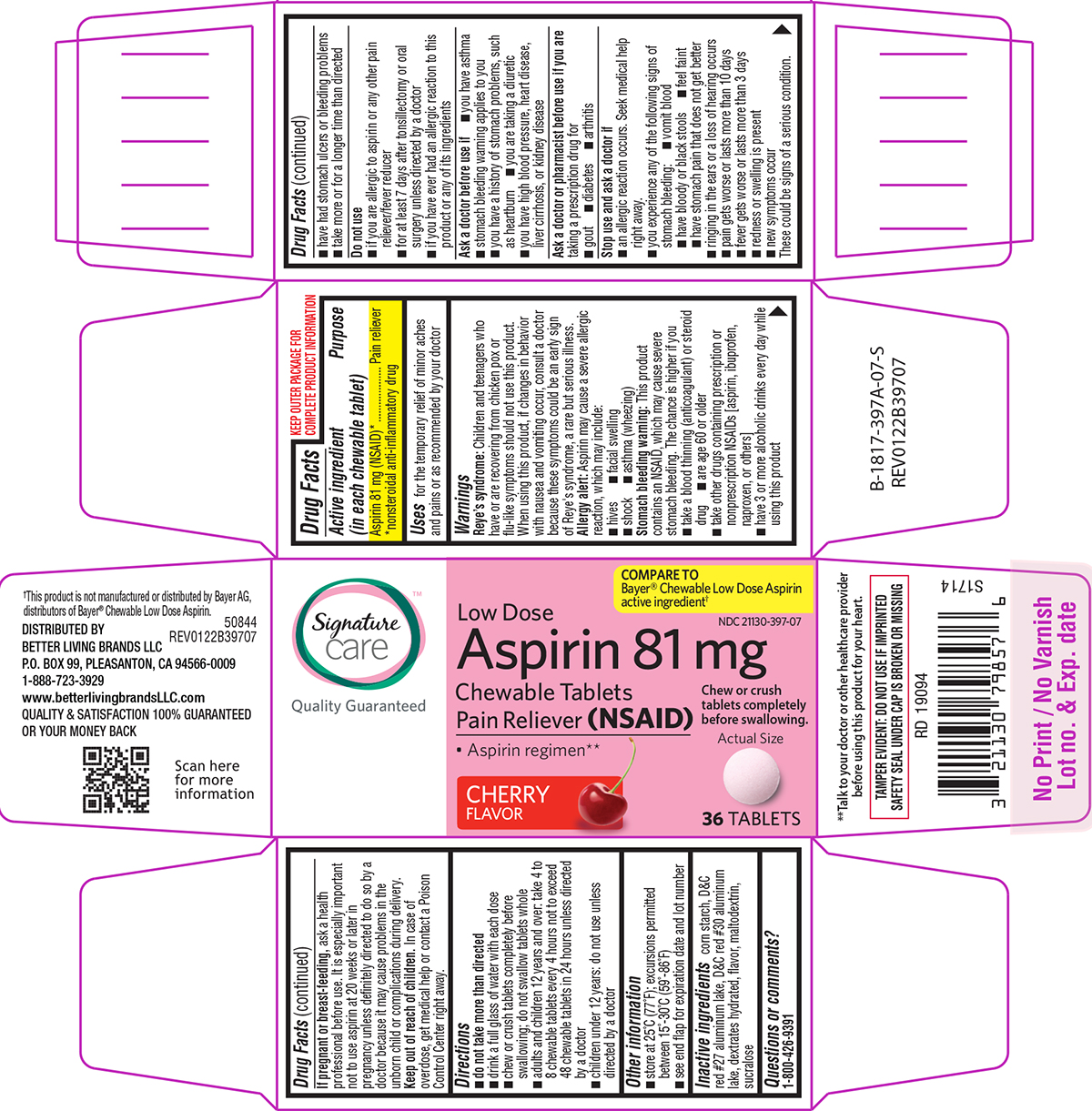
Label: ASPIRIN LOW DOSE CHEWABLE- aspirin tablet, chewable
- NDC Code(s): 21130-397-07
- Packager: Better Living Brands, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each chewable tablet)
- Purpose
- Uses
-
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction, which may include:
- hives
- facial swelling
- shock
- asthma (wheezing)
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- take a blood thinning (anticoagulant) or steroid drug
- are age 60 or older
- take other drugs containing prescription or nonprescription NSAIDs [aspirin, ibuprofen, naproxen, or others]
- have 3 or more alcoholic drinks every day while using this product
- have had stomach ulcers or bleeding problems
- take more or for a longer time than directed
Do not use
- if you are allergic to aspirin or any other pain reliever/fever reducer
- for at least 7 days after tonsillectomy or oral surgery unless directed by a doctor
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if
- you have asthma
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you are taking a diuretic
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
Ask a doctor or pharmacist before use if you are
taking a prescription drug for
- gout
- diabetes
- arthritis
Stop use and ask a doctor if
- an allergic reaction occurs. Seek medical help right away.
- you experience any of the following signs of stomach bleeding:
- vomit blood
- have bloody or black stools
- feel faint
- have stomach pain that does not get better
- vomit blood
- ringing in the ears or a loss of hearing occurs
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
These could be signs of a serious condition.
-
Directions
- do not take more than directed
- drink a full glass of water with each dose
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- adults and children 12 years and over: take 4 to 8 chewable tablets every 4 hours not to exceed 48 chewable tablets in 24 hours unless directed by a doctor
- children under 12 years: do not use unless directed by a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
Signature™
care
Quality GuaranteedCOMPARE TO
Bayer® Chewable Low Dose Aspirin
active ingredient†NDC 21130-397-07
Low Dose
Aspirin 81 mg
Chewable Tablets
Pain Reliever (NSAID)Chew or crush
tablets completely
before swallowing.• Aspirin regimen**
CHERRY
FLAVORActual Size
36 TABLETS
**Talk to your doctor or other healthcare provider
before using this product for your heart.TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING†This product is not manufactured or distributed by Bayer AG,
distributors of Bayer® Chewable Low Dose Aspirin.50844
REV0122B39707DISTRIBUTED BY
BETTER LIVING BRANDS LLC
P.O. BOX 99, PLEASANTON, CA 94566-0009
1-888-723-3929
www.betterlivingbrandsLLC.com
QUALITY & SATISFACTION 100% GUARANTEED
OR YOUR MONEY BACK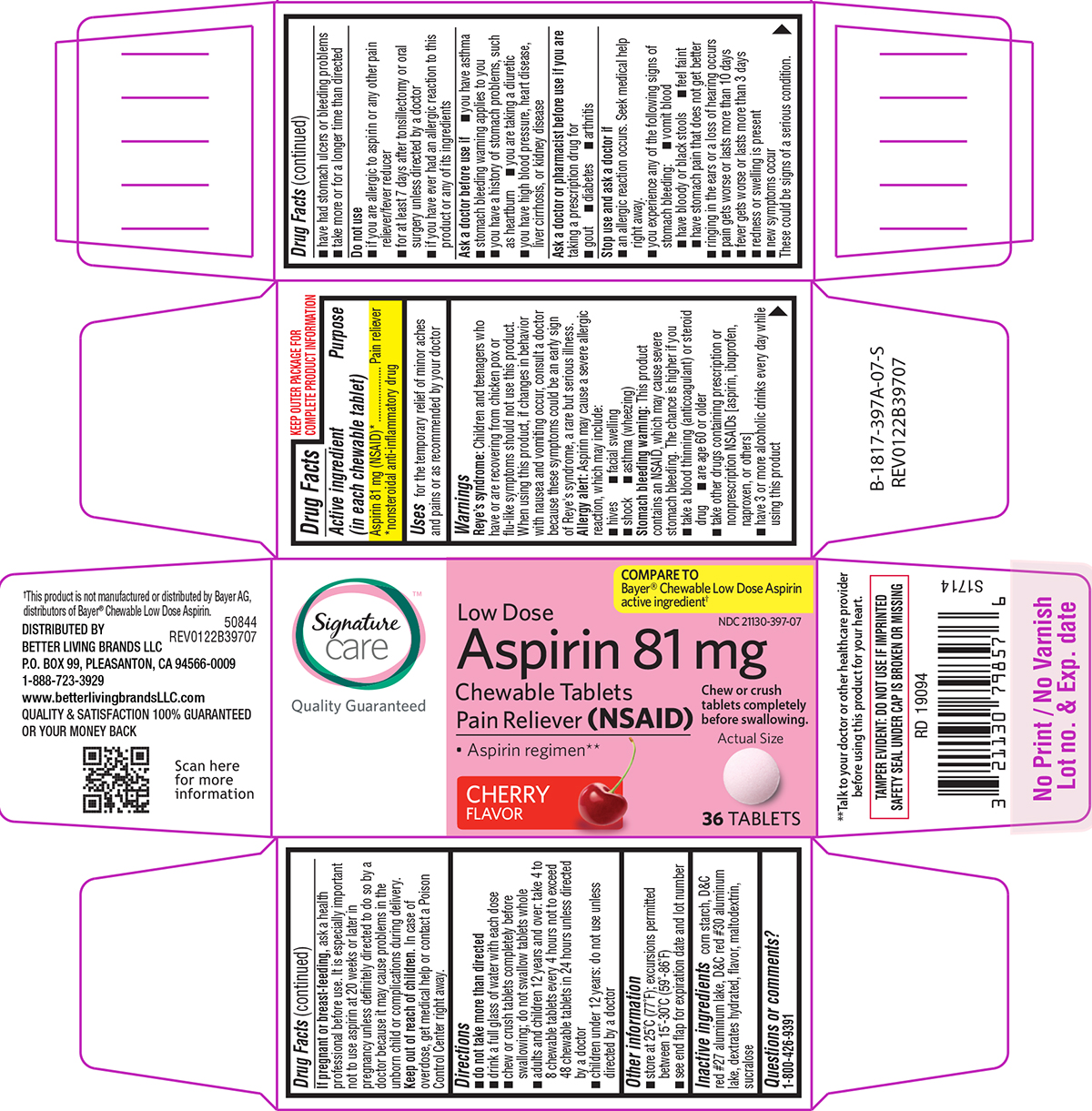
Signature Care 44-397A
-
INGREDIENTS AND APPEARANCE
ASPIRIN LOW DOSE CHEWABLE
aspirin tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21130-397 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 81 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) D&C RED NO. 30 (UNII: 2S42T2808B) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) MALTODEXTRIN (UNII: 7CVR7L4A2D) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color pink Score no score Shape ROUND Size 8mm Flavor CHERRY Imprint Code 44;397 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21130-397-07 1 in 1 CARTON 09/04/2001 1 36 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 09/04/2001 Labeler - Better Living Brands, LLC (009137209) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(21130-397) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(21130-397) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 manufacture(21130-397) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(21130-397) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(21130-397)