Label: PSORIASIS- niccolum sulphuricum, natrum bromatum, zincum bromatum, kali bromatum, kali sulphuricum. liquid
- NDC Code(s): 61480-105-03
- Packager: PLYMOUTH HEALTHCARE PRODUCTS LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 29, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
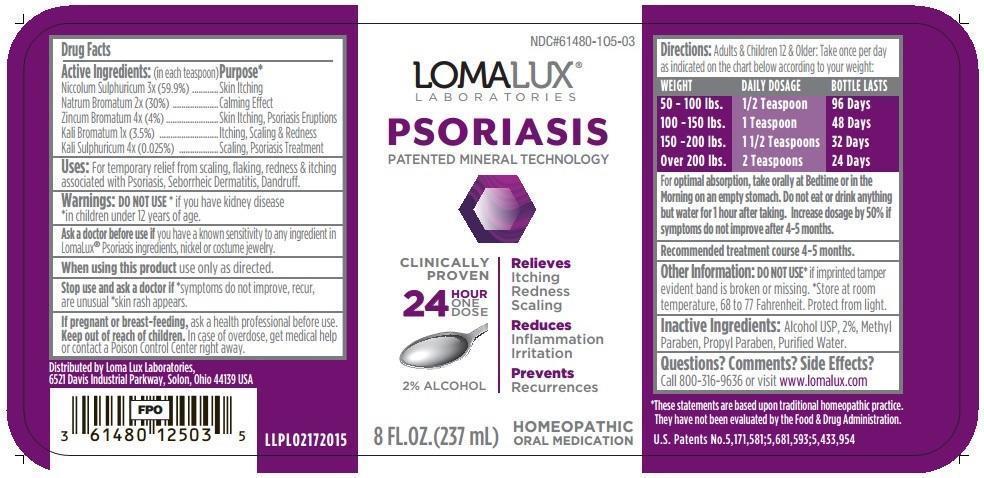
Drug Facts
Active Ingredients (in each teaspoon): Purpose* Niccolum Sulphuricum 3x (59.9%) Skin Itching Natrum Bromatum 2x (30%) Calming Effect Zincum Bromatum 4x (4%) Skin Itching, Psoriasis Eruptions Kali Bromatum 1x (3.5%) Itching, Scaling & Redness Kali Sulphuricum 4x (0.025%) Scaling, Psoriasis Treatment - INDICATIONS & USAGE
-
WARNINGS
Warnings:
- DO NOT USE * if you have kidney disease *in children under 12 years of age.
- Ask a doctor before use if you have a known sensitivity to any ingredient in LomaLux® Psoriasis ingredients, nickel or costume jewelry.
- When using this product use only as directed.
- Stop use and ask a doctor if *symptoms do not improve, recur, are unusual *skin rash appears
- If pregnant or breast-feeding, ask a health professional before use.
-
DOSAGE & ADMINISTRATION
Directions: Adults & Children 12 & Older: Take once per day as indicated on the chart below according to your weight:
WEIGHT DAILY DOSAGE BOTTLE LASTS
50 - 100 lbs. 1/2 Teaspoon 96 Days
100 - 150 lbs. 1 Teaspoon 48 Days
150 - 200 lbs. 1 1/2 Teaspoons 32 Days
Over 200 lbs. 2 Teaspoons 24 Days
For Optimal Absorption, take orally at Bedtime or in the Morning on an empty stomach. Do not eat or drink anything but water for 1 hour after taking. Increase dosage by 50% if symptoms do not improve after 4-5 months.
Recommended treatment course 4-5 months.
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- OTHER SAFETY INFORMATION
-
SPL UNCLASSIFIED SECTION
Loma Lux founder, Dr. Steven A. Smith M.D. developed Loma Lux® Psoriasis as a natural treatment for his psoriasis patients. Extensive scientific research & rigorous testing led to this exclusive formulation that has helped psoriasis sufferers for decates.
Unlike many conventional creams and lotions which only treat the surface of the skin, LomaLux Psoriasis is taken internally to help attack psoriasis at its source, by helping to gently stimulate your body's own recovery response. To achieve maximum benefits & relief, a 4-5 month treatment plan is recommended.
Over a Decade of Safe, Effective Use with NO Known Side Effects.
Finally, Healthy Skin!
Countless Studies have documented the skin healing powers of minerals, including the world renowned Dead Sea for soothing relief of many skin conditions. LomaLux® Psoriasis contains many of the same minerals as found in the Dead Sea - for natural health & well-being. Join the thousands of LomaLux® Psoriasis users, who for decades have found natural relief.
5 POWERFUL ACTIVE INGREDIENTS to help fight Psoriasis & Seborrhea
In a Clinical Test: 85% of Psoriasis Patients Experienced Real Relief.
- Itching
- Redness
- Scaling
- Inflammation
- Irritation
CLINICAL STUDY DATA available at www.lomalux.com
U.S. Patents No.5,171,581;5,681,593;5,433,954
-
PRINCIPAL DISPLAY PANEL
NEW ITEM
NDC#61480-105-03
NATURE CREATED. DERMATOLOGIST PERFECTED.™
LOMALUX® LABORATORIES
PSORIASIS
PATENTED MINERAL TECHNOLOGY
CLINICALLY PROVEN
Relieves
Itching
Redness
Scaling
Reduces
Inflammation
Irritation
Prevents
Recurrences
24 HOUR ONE DOSE
2% Alcohol
8 FL. OZ. (237 mL)
HOMEOPATHIC ORAL MEDICATION

-
INGREDIENTS AND APPEARANCE
PSORIASIS
niccolum sulphuricum, natrum bromatum, zincum bromatum, kali bromatum, kali sulphuricum. liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61480-105 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NICKEL SULFATE HEXAHYDRATE (UNII: JC9WZ4FK68) (NICKEL CATION - UNII:OIS2CXW7AM) NICKEL SULFATE HEXAHYDRATE 3 [hp_X] in 237 mL SODIUM BROMIDE (UNII: LC1V549NOM) (BROMIDE ION - UNII:952902IX06) SODIUM BROMIDE 2 [hp_X] in 237 mL ZINC BROMIDE (UNII: OO7ZBU9703) (ZINC CATION - UNII:13S1S8SF37) ZINC BROMIDE 4 [hp_X] in 237 mL POTASSIUM BROMIDE (UNII: OSD78555ZM) (BROMIDE ION - UNII:952902IX06) POTASSIUM BROMIDE 1 [hp_X] in 237 mL POTASSIUM SULFATE (UNII: 1K573LC5TV) (SULFATE ION - UNII:7IS9N8KPMG) POTASSIUM SULFATE 4 [hp_X] in 237 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61480-105-03 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/12/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 12/12/2016 Labeler - PLYMOUTH HEALTHCARE PRODUCTS LLC (079330314)


