Label: URUSA- ursodeoxycholic acid capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 72689-0001-1 - Packager: OASIS TRADING
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 19, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
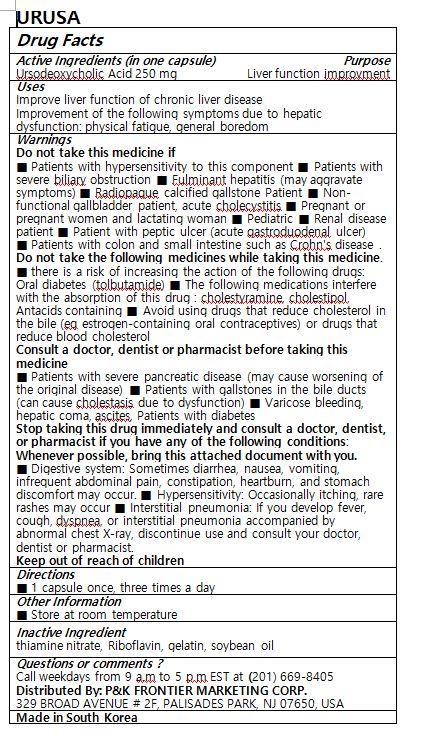
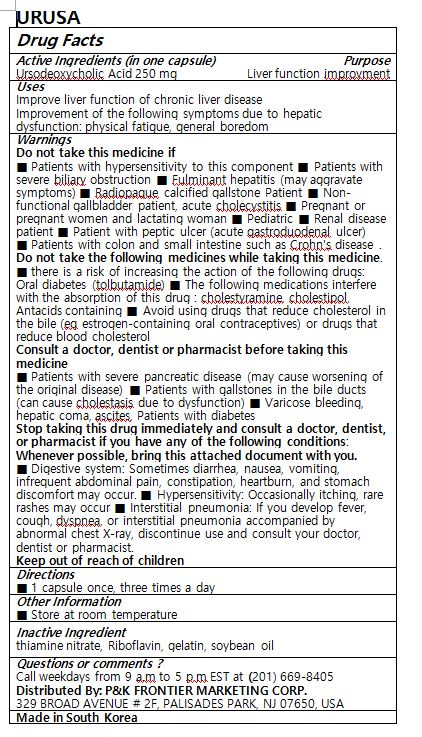
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Do not take this medicine if
■ Patients with hypersensitivity to this component ■ Patients with severe biliary obstruction ■ Fulminant hepatitis (may aggravate symptoms) ■ Radiopaque calcified gallstone Patient ■ Non-functional gallbladder patient, acute cholecystitis ■ Pregnant or pregnant women and lactating woman ■ Pediatric ■ Renal disease patient ■ Patient with peptic ulcer (acute gastroduodenal ulcer)
■ Patients with colon and small intestine such as Crohn's disease .
Do not take the following medicines while taking this medicine.
■ there is a risk of increasing the action of the following drugs: Oral diabetes (tolbutamide) ■ The following medications interfere with the absorption of this drug : cholestyramine, cholestipol, Antacids containing ■ Avoid using drugs that reduce cholesterol in the bile (eg estrogen-containing oral contraceptives) or drugs that reduce blood cholesterol
Consult a doctor, dentist or pharmacist before taking this medicine
■ Patients with severe pancreatic disease (may cause worsening of the original disease) ■ Patients with gallstones in the bile ducts (can cause cholestasis due to dysfunction) ■ Varicose bleeding, hepatic coma, ascites, Patients with diabetes
Stop taking this drug immediately and consult a doctor, dentist, or pharmacist if you have any of the following conditions: Whenever possible, bring this attached document with you.
■ Digestive system: Sometimes diarrhea, nausea, vomiting, infrequent abdominal pain, constipation, heartburn, and stomach discomfort may occur. ■ Hypersensitivity: Occasionally itching, rare rashes may occur ■ Interstitial pneumonia: If you develop fever, cough, dyspnea, or interstitial pneumonia accompanied by abnormal chest X-ray, discontinue use and consult your doctor, dentist or pharmacist.
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
URUSA
ursodeoxycholic acid capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72689-0001 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength URSODIOL (UNII: 724L30Y2QR) (URSODIOL - UNII:724L30Y2QR) URSODIOL 250 mg Inactive Ingredients Ingredient Name Strength RIBOFLAVIN (UNII: TLM2976OFR) GELATIN (UNII: 2G86QN327L) SOYBEAN OIL (UNII: 241ATL177A) THIAMINE MONONITRATE (UNII: 8K0I04919X) Product Characteristics Color yellow Score no score Shape OVAL Size 10mm Flavor Imprint Code URSA Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72689-0001-1 60 in 1 BLISTER PACK; Type 0: Not a Combination Product 11/16/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/15/2018 Labeler - OASIS TRADING (689991468) Registrant - OASIS TRADING (689991468) Establishment Name Address ID/FEI Business Operations OASIS TRADING 689991468 manufacture(72689-0001)