Label: ECOLAB- chloroxylenol solution
- NDC Code(s): 63146-124-10
- Packager: Kay Chemical Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 30, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
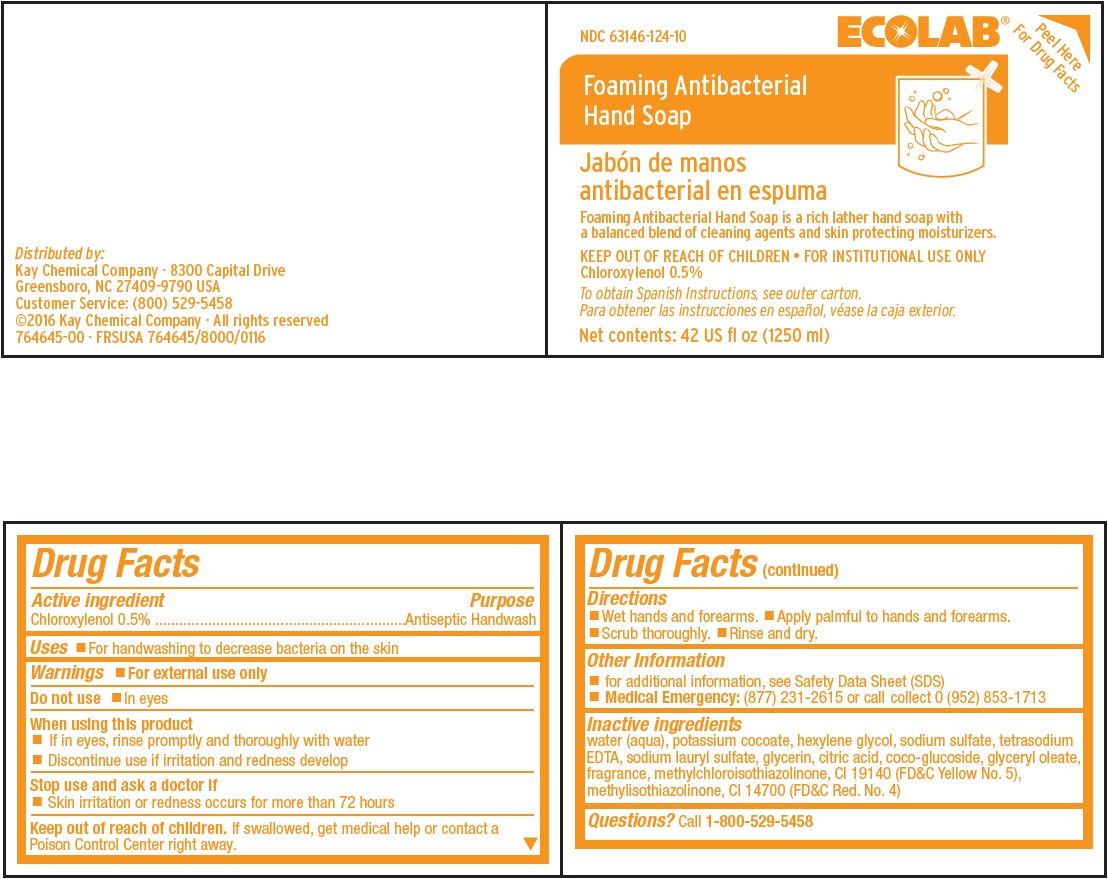
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
INACTIVE INGREDIENT
Inactive Ingredients
water (aqua), potassium cocoate, hexylene glycol, sodium sulfate, tetrasodium EDTA, sodium lauryl sulfate, glycerin, citric acid, coco-glucoside, glyceryl oleate, fragrance, methylchloroisothiazolinone, CI 19140 (FD&C Yellow No. 5), methylisothiazolinone, CI 14700 (FD&C Red. No. 4)
- QUESTIONS
-
Representative Label and Principal Display Panel
ECOLAB®
Peel Here For Drug Facts
NDC 63146-124-10
Foaming Antibacterial
Hand Soap
Jabón de manos
antibacterial en espumaFoaming Antibacterial Hand Soap is a rich lather hand soap with
a balanced blend of cleaning agents and skin protecting moisturizers.KEEP OUT OF REACH OF CHILDREN • FOR INSTITUTIONAL USE ONLY
Chloroxylenol 0.5%To obtain Spanish Instructions, see outer carton.
Para obtener las instrucciones en español, véase la caja exterior.Net contents: 42 US fl oz (1250 ml)
Distributed by:
Kay Chemical Company · 8300 Capital Drive
Greensboro, NC 27409-9790 USA
Customer Service: (800) 529-5458
©2016 Kay Chemical Company · All rights reserved
764645-00 · FRSUSA 764645/8000/0116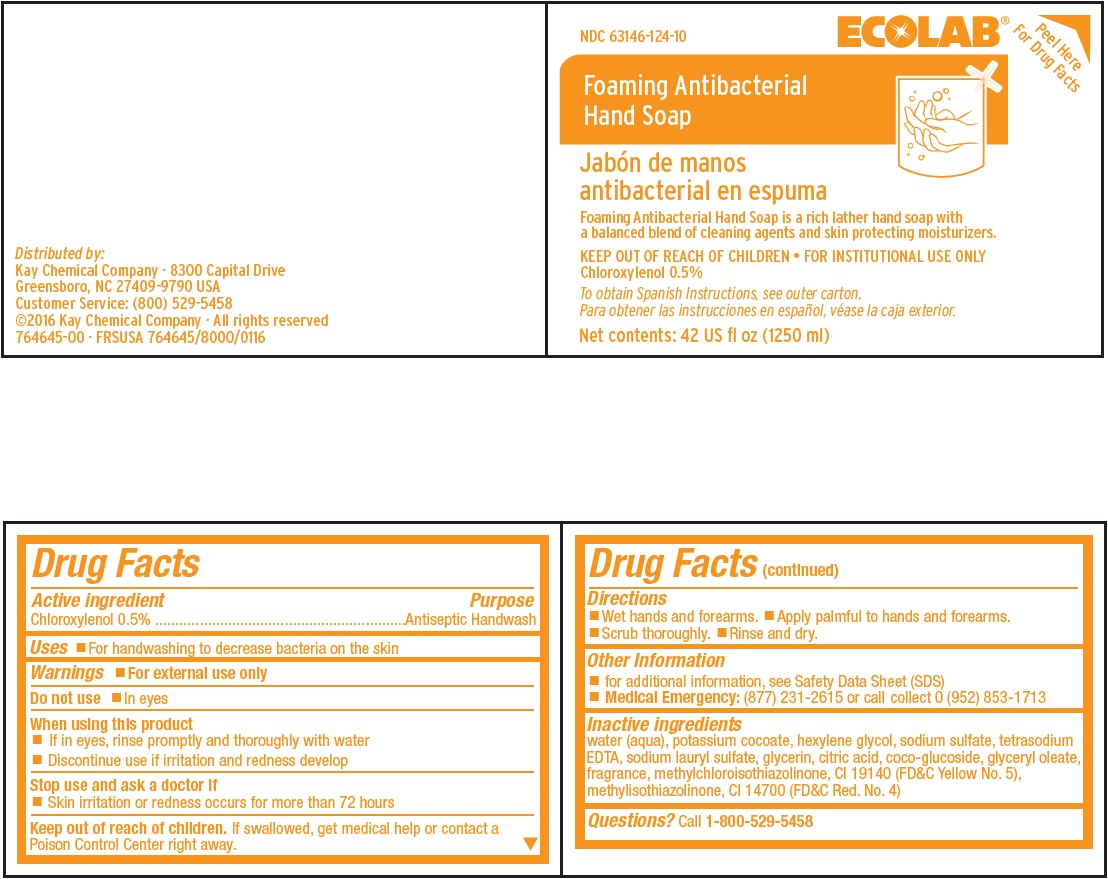
-
INGREDIENTS AND APPEARANCE
ECOLAB
chloroxylenol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63146-124 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POTASSIUM COCOATE (UNII: F8U72V8ZXP) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SODIUM SULFATE ANHYDROUS (UNII: 36KCS0R750) EDETATE SODIUM (UNII: MP1J8420LU) SODIUM LAURYL SULFATE (UNII: 368GB5141J) GLYCERIN (UNII: PDC6A3C0OX) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) COCO GLUCOSIDE (UNII: ICS790225B) GLYCERYL OLEATE (UNII: 4PC054V79P) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) FD&C RED NO. 4 (UNII: X3W0AM1JLX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63146-124-10 1250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/30/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 12/30/2015 Labeler - Kay Chemical Company (003237021)