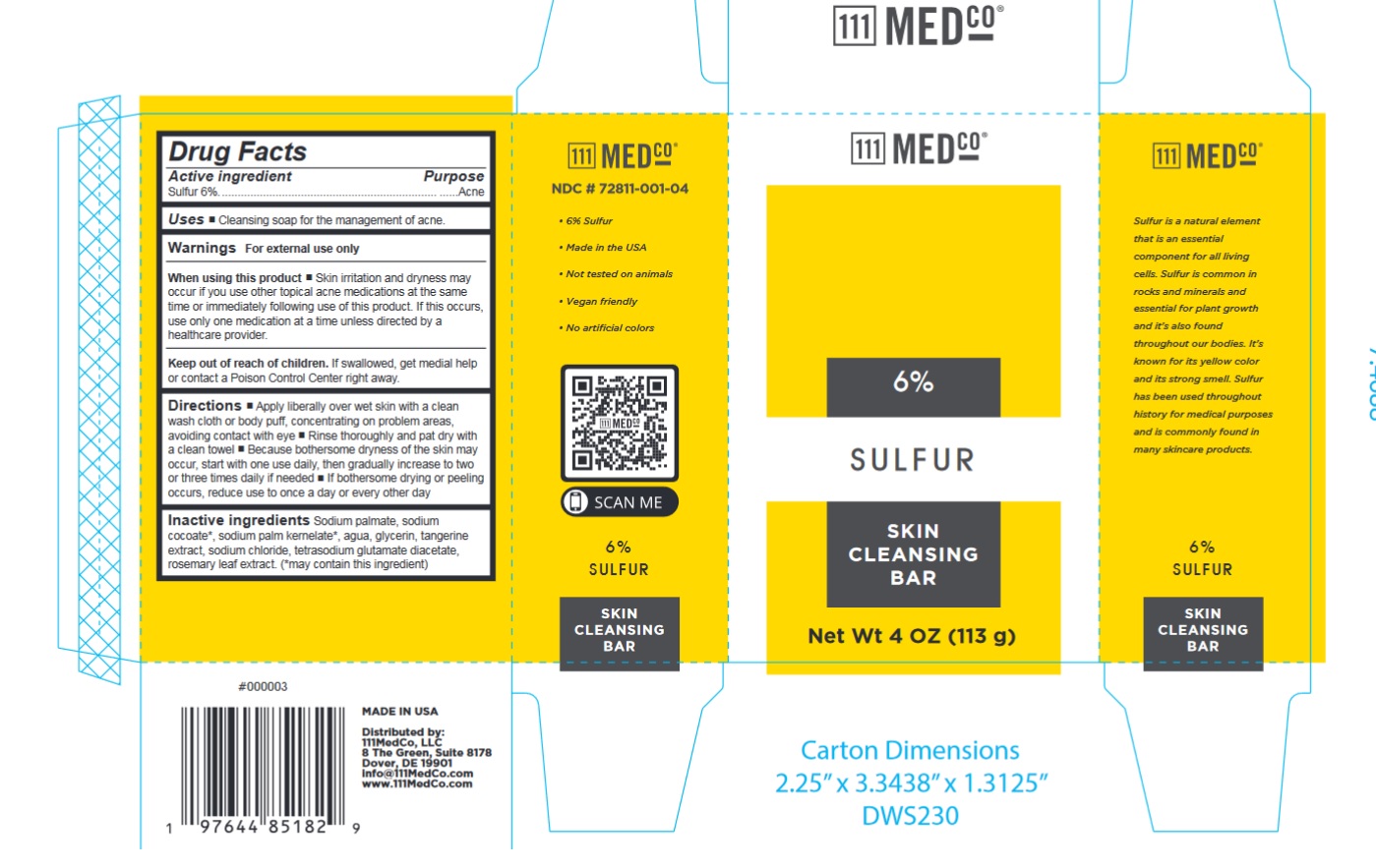
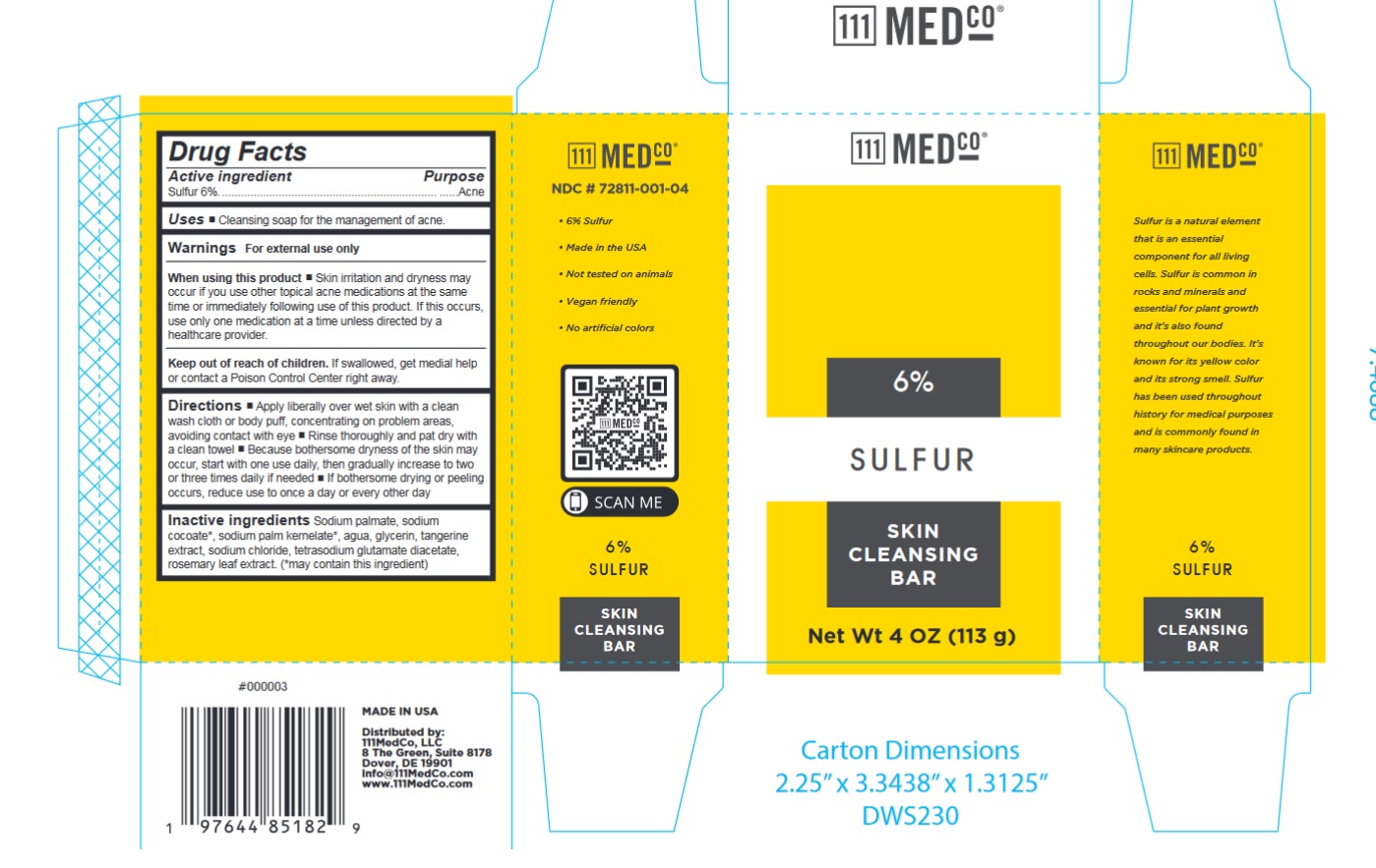
Label: 111MEDCO 6% SULFUR SKIN CLEANSING BAR- sulfur soap
- NDC Code(s): 72811-001-04
- Packager: 111MedCo LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
-
Directions
- Apply liberally over wet skin with a clean wash cloth or body puff, concentrating on problem areas, avoiding contact with eye
- Rinse thoroughly and pat dry with a clean towel
- Because bothersome dryness of the skin may occur, start with one use daily, then gradually increase to two or three times daily if needed
- If bothersome drying or peeling occurs, reduce use to once a day or every other day
- Inactive ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
111MEDCO 6% SULFUR SKIN CLEANSING BAR
sulfur soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72811-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 60 mg in 1 g Inactive Ingredients Ingredient Name Strength SODIUM PALMATE (UNII: S0A6004K3Z) SODIUM COCOATE (UNII: R1TQH25F4I) SODIUM PALM KERNELATE (UNII: 6H91L1NXTW) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) TANGERINE (UNII: KH3E3096OO) SODIUM CHLORIDE (UNII: 451W47IQ8X) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) ROSEMARY (UNII: IJ67X351P9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72811-001-04 113 g in 1 CARTON; Type 0: Not a Combination Product 09/06/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 09/06/2023 Labeler - 111MedCo LLC (065115643)