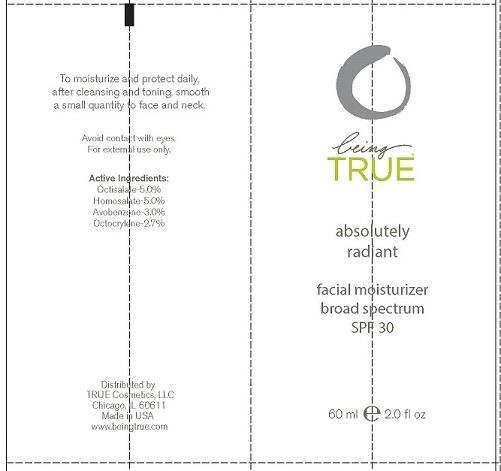
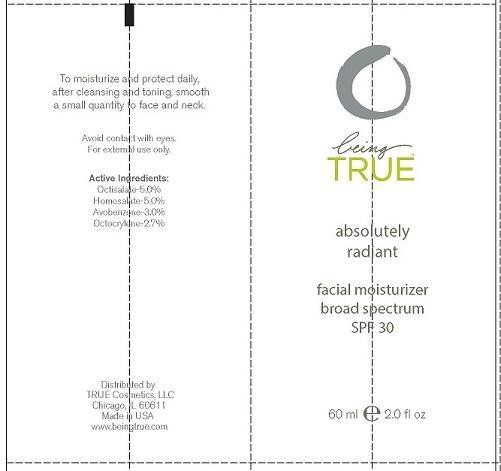
Label: BEING TRUE ESSENTIALS ABSOLUTELY RADIANT- octisalate, homosalate, avobenzone and octocrylene lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 60724-101-12, 60724-101-22 - Packager: TRUE Cosmetics LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 19, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
WARNINGS
SKN CANCER/SKIN AGING ALERT: SPENDING TIME IN THE SUN INCREASES YOU RISK OF SKIN CANCER AND EARLY SKIN AGING. THIS PRODUCT HAS BEEN SHOWN ONLY TO PREVENT SUNBURN, NOT SKIN CANCER OR EARLY AGING.
FOR EXTERNAL USE ONY.
DO NOT USE ON DAMAGED OR BROKEN SKIN.
WHEN USING THIS PRODUCT KEEP OUT OF EYES.
RINSE WITH WATER TO REMOVE.
STOP USE AND ASK A DOCTOR IF RASH OCCURS.
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
INACTIVE INGREDENTS
ALOE BARBADENSIS LEAF JUICE, ALOE BARBADENSIS LEAF EXTRACT, AMINOMETHYL PROPANOL, AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER, BUTYLENE GLYCOL, CAPRYLYL GLYCOL, CETEARYL ALCOHOL, CHLORPHENESIN, CITRUS SINENSIS (BLOOD ORANGE) FRUIT EXTRACT, COCO CAPRYLATE/CAPRATE, DECYL GLUCOSIDE, DEXTRIN, DIMETHICONE, DISODIUM EDTA, ETHYHEXYL STEARATE, HYDROLYZED HIBISCUS ESCULENTUS EXTRACT, ISODODECANE, PEG-100 STEARATE, PENTYLENE GLYCOL, PHENOXYETHANOL, POLYSILICONE-11, POLYURETHANE-34, SODIUM CITRATE, SODIUM POLYACRYLATE, TAPIOCA STARCH, POLYMETHYLSILSESQUIOXANE, TOCOPHERYL ACETATE, TRIDECETH 6, WATER, XANTHAN GUM.
- OTHER SAFETY INFORMATION
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BEING TRUE ESSENTIALS ABSOLUTELY RADIANT
octisalate, homosalate, avobenzone and octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:60724-101 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2.7 g in 100 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) CHLORPHENESIN (UNII: I670DAL4SZ) CITRUS SINENSIS WHOLE (UNII: 37673443KL) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) ICODEXTRIN (UNII: 2NX48Z0A9G) PEG-100 STEARATE (UNII: YD01N1999R) PENTYLENE GLYCOL (UNII: 50C1307PZG) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSILICONE-15 (UNII: F8DRP5BB29) POLYBUTENE (1400 MW) (UNII: 1NA5AO9GH7) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM POLYACRYLATE (8000 MW) (UNII: 285CYO341L) STARCH, TAPIOCA (UNII: 24SC3U704I) POLYMETHYLSILSESQUIOXANE (11 MICRONS) (UNII: Z570VEV8XK) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRIDECETH-6 (UNII: 3T5PCR2H0C) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:60724-101-22 1 in 1 PACKAGE 1 NDC:60724-101-12 60 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 09/19/2013 Labeler - TRUE Cosmetics LLC (621646913) Establishment Name Address ID/FEI Business Operations Northwest Cosmetic Laboratories LLC 929572014 manufacture(60724-101) , pack(60724-101)