Label: SIMETHICONE- dimethicone capsule, liquid filled
-
Contains inactivated NDC Code(s)
NDC Code(s): 68210-1901-1, 68210-1901-2, 68210-1901-3, 68210-1901-4, view more68210-1901-5 - Packager: SPIRIT PHARMACEUTICALS,LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 9, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (per softgel)
- Purpose
- Use
- Warnings
- Directions
- Other information
- Inactive ingredients
-
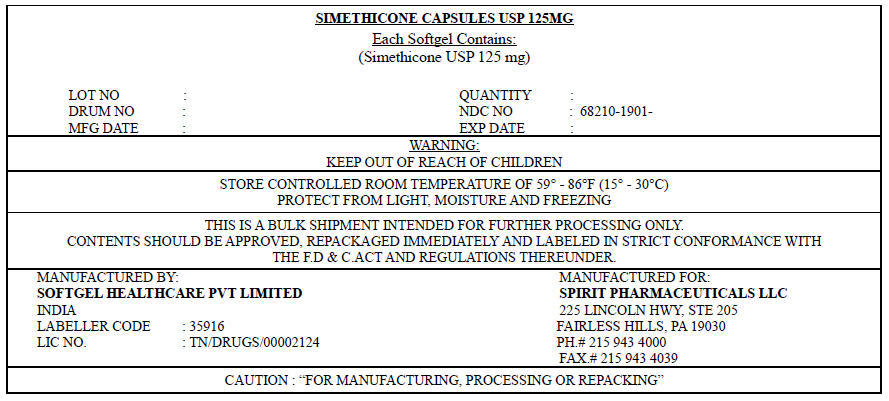
PRINCIPAL DISPLAY PANEL - 125 mg Label
SIMETHICONE CAPSULES USP 125MG
Each Softgel Contains:
(Simethicone USP 125 mg)LOT NO : QUANTITY : DRUM NO : NDC NO : 68210-1901- MFG DATE : EXP DATE : WARNING:
KEEP OUT OF REACH OF CHILDRENSTORE CONTROLLED ROOM TEMPERATURE OF 59° – 86°F (15° – 30°C)
PROTECT FROM LIGHT, MOISTURE AND FREEZINGTHIS IS A BULK SHIPMENT INTENDED FOR FURTHER PROCESSING ONLY.
CONTENTS SHOULD BE APPROVED, REPACKAGED IMMEDIATELY AND LABELED IN STRICT
CONFORMANCE WITH THE F.D&C ACT AND REGULATIONS THEREUNDER.MANUFACTURED BY:
SOFTGEL HEALTHCARE PVT LIMITED
INDIA
LABELLER CODE : 35916
LIC NO. : TN/DRUGS/00002124MANUFACTURED FOR:
SPIRIT PHARMACEUTICALS LLC
225 LINCOLN HWY, STE 205
FAIRLESS HILLS, PA 19030
PH.# 215 943 4000
FAX.# 215 943 4039CAUTION : "FOR MANUFACTURING, PROCESSING OR REPACKING"
-
INGREDIENTS AND APPEARANCE
SIMETHICONE
dimethicone capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68210-1901 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 125 mg Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) GELATIN (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) PEPPERMINT OIL (UNII: AV092KU4JH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) Product Characteristics Color GREEN Score no score Shape OVAL Size 10mm Flavor Imprint Code P10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68210-1901-1 10 in 1 BOX 2 NDC:68210-1901-2 30 in 1 BOX 3 NDC:68210-1901-3 1000 in 1 BOX 4 NDC:68210-1901-4 16000 in 1 BOX 5 NDC:68210-1901-5 20000 in 1 BOX Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part332 03/01/2010 Labeler - SPIRIT PHARMACEUTICALS,LLC (179621011) Establishment Name Address ID/FEI Business Operations SOFTGEL HEALTHCARE PVT LTD 675584180 MANUFACTURE
