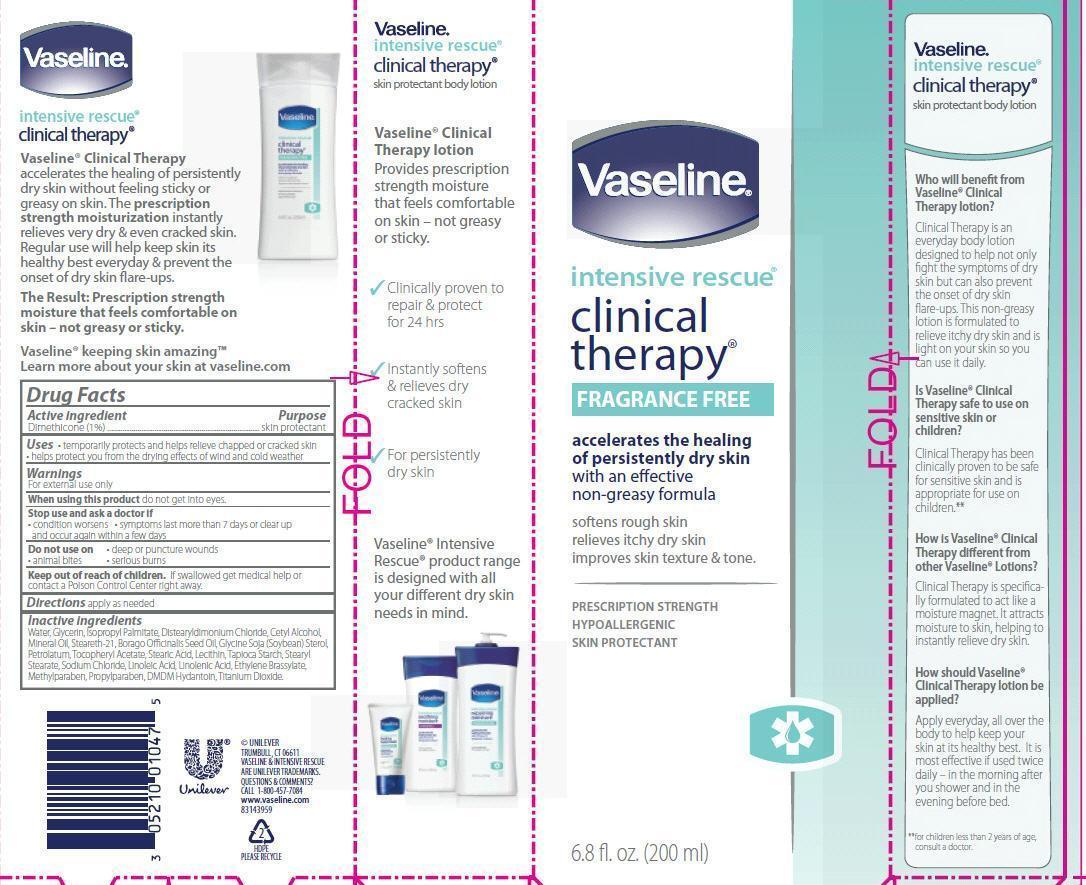
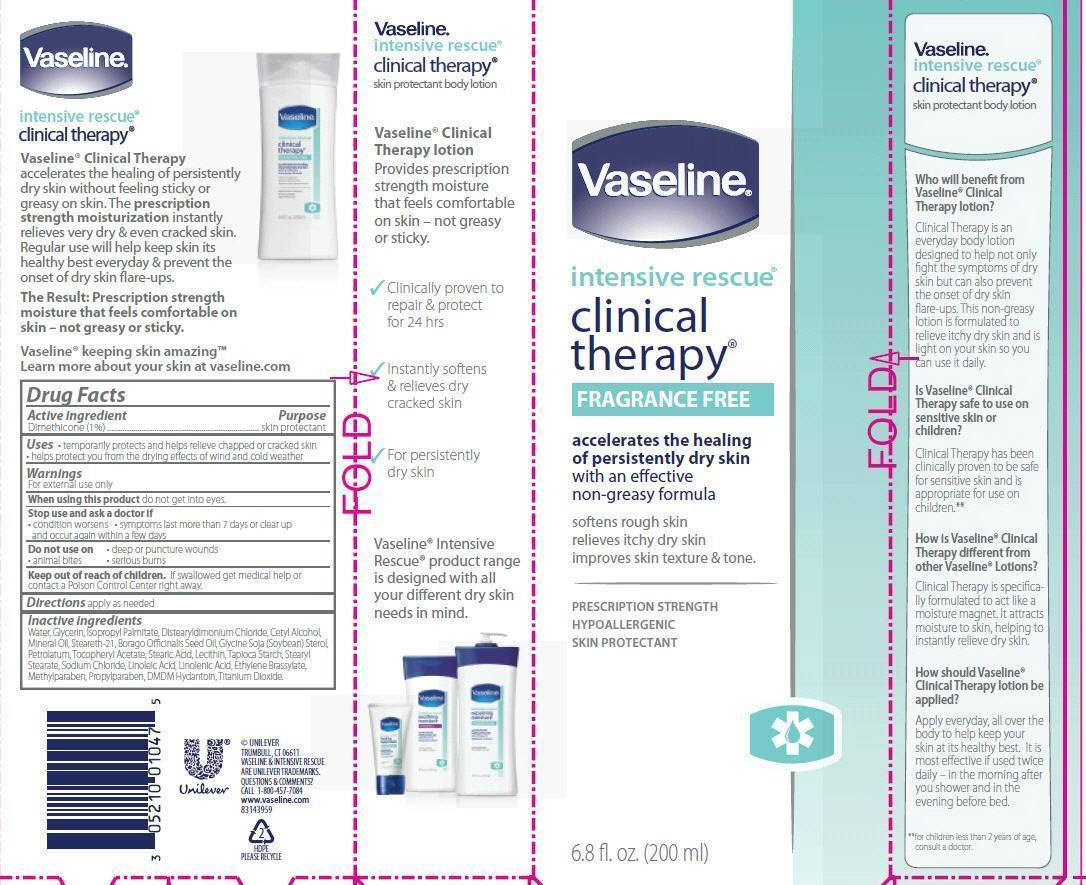
Label: VASELINE INTENSIVE RESCUE CLINICAL THERAPY FRAGRANCE FREE- dimethicone lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 64942-1038-1, 64942-1038-2, 64942-1038-3 - Packager: Conopco Inc. d/b/a Unilever
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 15, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
-
INACTIVE INGREDIENT
Inactive ingredients
Water, Glycerin, Isopropyl Palmitate, Distearyldimonium Chloride, Cetyl Alcohol, Mineral Oil, Steareth-21, Borago Officinalis Seed Oil, Glycine Soja (Soybean) Sterol, Petrolatum, Tocopheryl Acetate, Stearic Acid, Lecithin, Tapioca Starch, Stearyl Stearate, Sodium Chloride, Linoleic Acid, Linolenic Acid, Ethylene Brassylate, Methylparaben, Propylparaben, DMDM Hydantoin, Titanium Dioxide.
- QUESTIONS
- PDP 6.8 fl. oz.
- 6.8 fl. oz. carton
-
INGREDIENTS AND APPEARANCE
VASELINE INTENSIVE RESCUE CLINICAL THERAPY FRAGRANCE FREE
dimethicone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64942-1038 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) DISTEARYLDIMONIUM CHLORIDE (UNII: OM9573ZX3X) CETYL ALCOHOL (UNII: 936JST6JCN) MINERAL OIL (UNII: T5L8T28FGP) STEARETH-21 (UNII: 53J3F32P58) BORAGO OFFICINALIS SEED (UNII: 2GXJ790US0) PETROLATUM (UNII: 4T6H12BN9U) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) STEARIC ACID (UNII: 4ELV7Z65AP) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) STARCH, TAPIOCA (UNII: 24SC3U704I) STEARYL STEARATE (UNII: 5WX2EGD0DK) SODIUM CHLORIDE (UNII: 451W47IQ8X) LINOLEIC ACID (UNII: 9KJL21T0QJ) LINOLENIC ACID (UNII: 0RBV727H71) DMDM HYDANTOIN (UNII: BYR0546TOW) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64942-1038-2 1 in 1 CARTON 1 NDC:64942-1038-1 200 mL in 1 BOTTLE, PLASTIC 2 NDC:64942-1038-3 50 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part347 06/23/2008 Labeler - Conopco Inc. d/b/a Unilever (001375088)