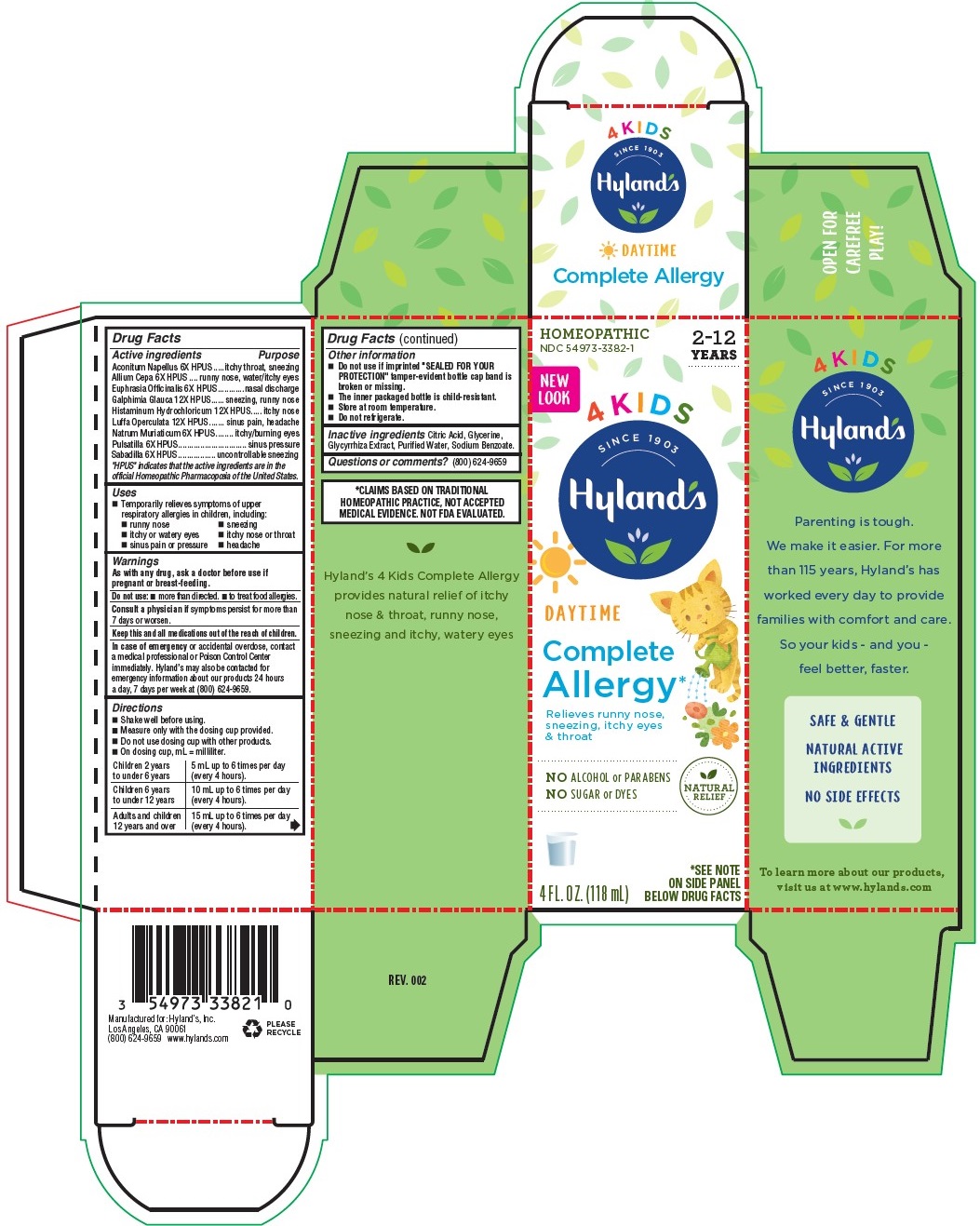
Label: 4 KIDS COMPLETE ALLERGY DAYTIME- sodium chloride, aconitum napellus, luffa operculata fruit, euphrasia stricta,galphimia glauca flowering top, histamine dihydrochloride,onion,anemone pulsatilla and schoennocaulon officinale seed liquid
- NDC Code(s): 54973-3382-1
- Packager: Hyland's Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 22, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Active ingredients
Aconitum Napellus 6X HPUS
Allium Cepa 6X HPUS
Euphrasia Officinalis 6X HPUS
Galphimia Glauca 12X HPUS
Histaminum Hydrochloricum 12X HPUS
Luffa Operculata 12X HPUS
Natrum Muriaticum 6X HPUS
Pulsatilla 6X HPUS
Sabadilla 6X HPUS
“HPUS” indicates that the active ingredients are in the
official Homeopathic Pharmacopœia of the United States.
Purpose
Aconitum Napellus 6X HPUS ....itchy throat, sneezing
Allium Cepa 6X HPUS ..runny nose, watery/itchy eyes
Euphrasia Officinalis 6X HPUS...........nasal discharge
Galphimia Glauca 12X HPUS .... sneezing, runny nose
Histaminum Hydrochloricum 12X HPUS ... itchy nose
Luffa Operculata 12X HPUS .......sinus pain, headache
Natrum Muriaticum 6X HPUS ....... itchy/burning eyes
Pulsatilla 6X HPUS.............................. sinus pressure
Sabadilla 6X HPUS ................. uncontrollable sneezing
- Uses
- Warnings
-
Directions
■ Shake well before using.
■ Measure only with the dosing cup provided.
■ Do not use dosing cup with other products.
■ On dosing cup, mL = milliliter.
Children 2 years
to under 6 years
5 mL up to 6 times per day
(every 4 hours).
Children 6 years
to under 12 years
10 mL up to 6 times per day
(every 4 hours).
Adults and children
12 years and over
15 mL up to 6 times per day
(every 4 hours).
- Drug Facts (continued)
- Inactive ingredients
- Questions or comments?
- *CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE, NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
4 KIDS COMPLETE ALLERGY DAYTIME
sodium chloride, aconitum napellus, luffa operculata fruit, euphrasia stricta,galphimia glauca flowering top, histamine dihydrochloride,onion,anemone pulsatilla and schoennocaulon officinale seed liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54973-3382 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SCHOENOCAULON OFFICINALE SEED (UNII: 6NAF1689IO) (SCHOENOCAULON OFFICINALE SEED - UNII:6NAF1689IO) SCHOENOCAULON OFFICINALE SEED 6 [hp_X] in 1 mL ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 6 [hp_X] in 1 mL ANEMONE PULSATILLA (UNII: I76KB35JEV) (ANEMONE PULSATILLA - UNII:I76KB35JEV) ANEMONE PULSATILLA 6 [hp_X] in 1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 6 [hp_X] in 1 mL ACONITUM NAPELLUS (UNII: U0NQ8555JD) (ACONITUM NAPELLUS - UNII:U0NQ8555JD) ACONITUM NAPELLUS 6 [hp_X] in 1 mL EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 6 [hp_X] in 1 mL GALPHIMIA GLAUCA FLOWERING TOP (UNII: 93PH5Q8M7E) (GALPHIMIA GLAUCA FLOWERING TOP - UNII:93PH5Q8M7E) GALPHIMIA GLAUCA FLOWERING TOP 12 [hp_X] in 1 mL HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 12 [hp_X] in 1 mL LUFFA OPERCULATA FRUIT (UNII: C4MO6809HU) (LUFFA OPERCULATA FRUIT - UNII:C4MO6809HU) LUFFA OPERCULATA FRUIT 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54973-3382-1 1 in 1 CARTON 11/01/2018 1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/01/2018 Labeler - Hyland's Inc. (008316655)