Label: APOTHECARE- salicylic acid liquid
- NDC Code(s): 64942-1627-1
- Packager: Conopco, Inc. d/b/a/ Unilever
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 7, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
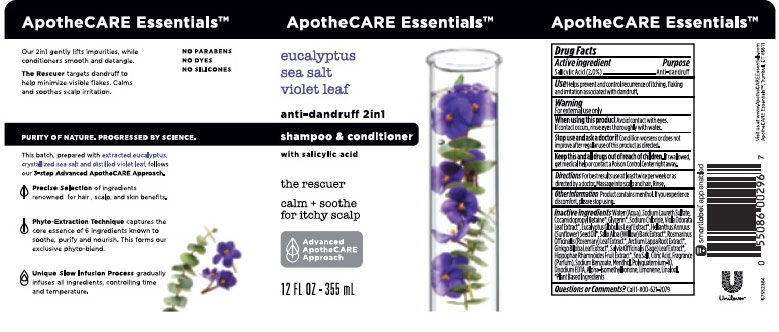
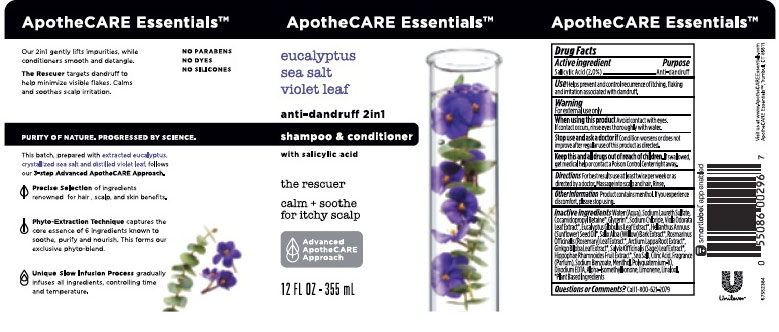
- APOTHECARE ESSENTIALS EUCALYPTUS SEA SALT VIOLET LEAF 2IN1 ANTI-DANDRUFF SHAMPOO & CONDITIONER - salicylic acid emulsion
- Drug Facts
- Purpose
- Use
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other Information
-
Inactive Ingredients
Water (Aqua), Sodium Laureth Sulfate, Cocamidopropyl Betaine, Glycerin, Sodium Chloride, Viola Odorata Leaf Extract, Eucalyptus Globulus Leaf Extract, Helianthus Annuus (Sunflower) Seed Oil, Salix Alba (Willow) Bark Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Arctium Lappa Root Extract,Ginkgo Biloba Leaf Extract, Salvia Officinalis (Sage) Leaf Extract, Hippophae Rhamnoides Fruit Extract, Sea Salt, Citric Acid, Fragrance (Parfum), Sodium Benzoate, Menthol, Polyquaternium-10, Disodium EDTA, Alpha-Isomethyl Ionone, Limonene, Linalool.
- Questions, comments?
- Packaging
-
INGREDIENTS AND APPEARANCE
APOTHECARE
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64942-1627 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 2 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) SODIUM CHLORIDE (UNII: 451W47IQ8X) SUNFLOWER OIL (UNII: 3W1JG795YI) GLYCERIN (UNII: PDC6A3C0OX) ARCTIUM LAPPA ROOT (UNII: 597E9BI3Z3) GINKGO (UNII: 19FUJ2C58T) SEA SALT (UNII: 87GE52P74G) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM BENZOATE (UNII: OJ245FE5EU) MENTHOL (UNII: L7T10EIP3A) POLYQUATERNIUM-10 (1000 MPA.S AT 2%) (UNII: GMR4PEN8PK) EDETATE DISODIUM (UNII: 7FLD91C86K) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64942-1627-1 355 mL in 1 CONTAINER; Type 0: Not a Combination Product 01/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part358H 01/01/2020 Labeler - Conopco, Inc. d/b/a/ Unilever (001375088)