Label: OYSTER SHELL CALCIUM- calcium carbonate tablet
- NHRIC Code(s): 76413-322-01
- Packager: Central Texas Community Health Centers
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated September 15, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
Supplement Facts Serving Size: 1 Tablet Amount Per Serving % Daily Value Calcium (as Oyster Shell) 500 mg. 50% OTHER INGREDIENTS: Croscarmellose Sodium, Microcrystalline Cellulose, Hypromellose, Polydextrose, Titanium Dioxide, Soy Polysaccharide, Talc, Sodium Lauryl Sulfate and Triglycerides.
Contains: Soy and Shellfish
- DIRECTIONS
- WARNING
- FREE OF
- HEALTH CLAIM
- SAFE HANDLING WARNING
- HEALTH CLAIM
-
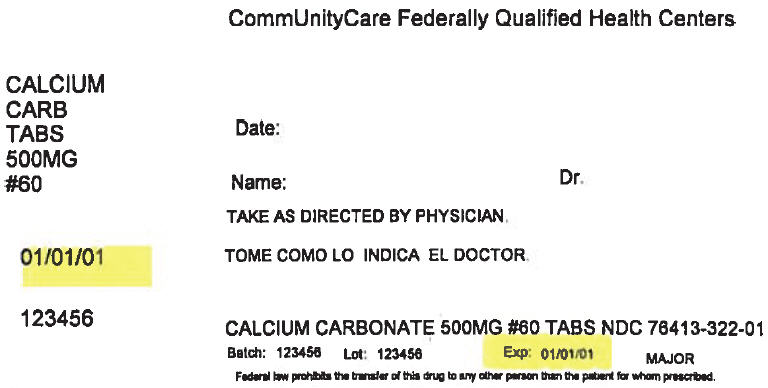
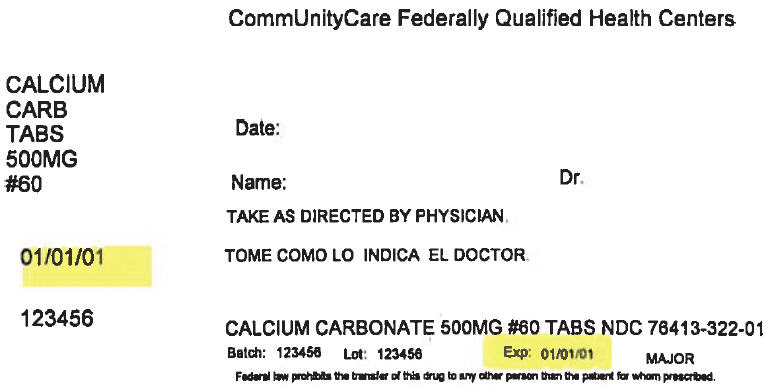
PRINCIPAL DISPLAY PANEL - 500 mg Tablet Bottle Label
CommUnityCare Federally Qualified Health Centers
CALCIUM
CARB
TABS
500MG
#60Date:
Name:
Dr.TAKE AS DIRECTED BY PHYSICIAN.
01/01/01
123456
CALCIUM CARBONATE 500MG #60 TABS NDC 76413-322-01
Batch:
123456
Lot:
123456
Exp:
01/01/01MAJOR
Federal law prohibits the transfer of this drug to any other person than the patient for whom prescribed.
-
INGREDIENTS AND APPEARANCE
OYSTER SHELL CALCIUM
calcium carbonate tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:76413-322 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Calcium carbonate (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 500 mg Inactive Ingredients Ingredient Name Strength Croscarmellose sodium (UNII: M28OL1HH48) Microcrystalline Cellulose (UNII: OP1R32D61U) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) Polydextrose (UNII: VH2XOU12IE) Titanium Dioxide (UNII: 15FIX9V2JP) Talc (UNII: 7SEV7J4R1U) Sodium Lauryl Sulfate (UNII: 368GB5141J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:76413-322-01 60 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 04/01/2016 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 1 mm Labeler - Central Texas Community Health Centers (079674019) Establishment Name Address ID/FEI Business Operations Central Texas Community Health Centers 079674019 REPACK, RELABEL Establishment Name Address ID/FEI Business Operations Major Pharmaceuticals 383359612 MANUFACTURE