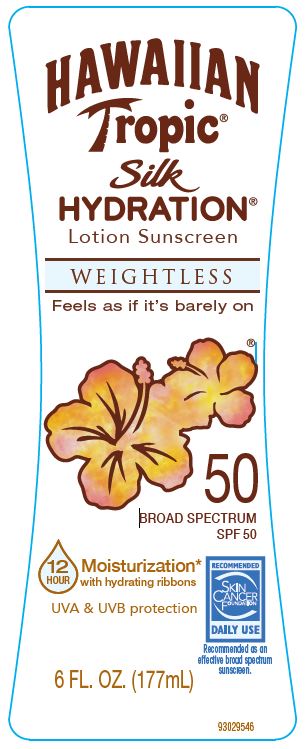
Label: HAWAIIAN TROPIC- avobenzone, homosalate, octisalate, octocrylene lotion
- NDC Code(s): 63354-420-54
- Packager: Edgewell Personal Care Brands LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Uses
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor
- Keep out of reach of children
-
Directions
Apply liberally 15 minutes before sun exposure • reapply: • after 80 minutes of swimming or sweating • immediately after towel drying. • at least every 2 hours
Sun Protection Measures Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF value of 15 or higher and other sun protection measures including: • limit time in the sun, especially from 10 a.m. - 2 p.m. • wear long-sleeved shirts, pants, hats, and sunglasses.•children under 6 months: Ask a doctor.
-
Inactive Ingredients
Water, Isohexadecane, Diisopropyl Adipate, C12-15 Alkyl Benzoate, Butylene Glycol, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Cetearyl Alcohol, Acrylates/C12-22 Alkyl Methacrylate Copolymer, Phenoxyethanol, Caprylyl Glycol, Fragrance, Methylparaben, Ceteth-10 Phosphate, Dicetyl Phosphate, Propylparaben, Polysorbate 60, Disodium EDTA, Xanthan Gum, Aminomethyl Propanol, Mica, Tocopheryl Acetate, Titanium Dioxide, Panthenol, Aloe Barbadensis Leaf Juice, Silk Amino Acids, Sodium Ascorbyl Phosphate, Carica Papaya (Papaya) Fruit Extract, Colocasia Antiquorum Root Extract, Mangifera Indica (Mango) Fruit Extract, Passiflora Incarnata Fruit Extract, Plumeria Acutifolia Flower Extract, Psidium Guajava Fruit Extract, Iron Oxides.
- Other Information
- Questions or Comments?
- Purpose
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HAWAIIAN TROPIC
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63354-420 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.7 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 5 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 8 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 g in 100 g Inactive Ingredients Ingredient Name Strength XANTHAN GUM (UNII: TTV12P4NEE) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) BROWN IRON OXIDE (UNII: 1N032N7MFO) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PANTHENOL (UNII: WV9CM0O67Z) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) COLOCASIA ESCULENTA ROOT (UNII: H7B71Q0G0D) PASSIFLORA INCARNATA FRUIT (UNII: SF206I8G4P) PLUMERIA RUBRA FLOWER (UNII: 8P7XXY759H) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) EDETATE DISODIUM (UNII: 7FLD91C86K) ISOHEXADECANE (UNII: 918X1OUF1E) AMINO ACIDS, SILK (UNII: V0L00EX1IA) WATER (UNII: 059QF0KO0R) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) PROPYLPARABEN (UNII: Z8IX2SC1OH) POLYSORBATE 60 (UNII: CAL22UVI4M) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) METHYLPARABEN (UNII: A2I8C7HI9T) CETETH-10 PHOSPHATE (UNII: 4E05O5N49G) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) ALOE VERA LEAF (UNII: ZY81Z83H0X) CARICA PAPAYA WHOLE (UNII: S0U63B0Q51) MANGIFERA INDICA WHOLE (UNII: U34MFR958F) PSIDIUM GUAJAVA WHOLE (UNII: 4J570BB45U) MICA (UNII: V8A1AW0880) DIHEXADECYL PHOSPHATE (UNII: 2V6E5WN99N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63354-420-54 177 g in 1 BOTTLE; Type 0: Not a Combination Product 10/29/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 10/29/2018 Labeler - Edgewell Personal Care Brands LLC (151179769)