Label: EAZI-BREED CIDR CATTLE INSERT- progesterone insert, extended release
- NDC Code(s): 54771-5207-1
- Packager: Zoetis Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated June 14, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
EAZI-BREED™
CIDR®
(progesterone intravaginal insert)
Cattle Insert
Each EAZI-BREED CIDR Cattle Insert contains 1.38 grams of progesterone in molded silicone over a flexible nylon spine. Attached to each EAZI-BREED CIDR Cattle Insert is a polyester tail.
NET CONTENTS
10 EAZI-BREED CIDR Cattle Inserts per bag
Approved by FDA under NADA # 141-200 - DRUG FACTS
-
Uses
• Synchronization of estrus in lactating dairy cows, suckled beef cows, and replacement beef and dairy heifers
• Induction of estrous cycles in anestrous lactating dairy cows
• Synchronization of the return to estrus in lactating dairy cows inseminated at the immediately preceding estrus
• Advancement of first postpartum estrus in suckled beef cows
• Advancement of first pubertal estrus in replacement beef heifers
Removal of the EAZI-BREED CIDR Cattle Insert on treatment Day 7 results in a rapid fall in plasma progesterone levels, which results in the occurrence of estrus in those animals responding to treatment. -
WARNINGS
Withdrawal Periods: Neither a pre-slaughter withdrawal interval nor a milk discard time is required when this product is used according to label directions.
User Safety Warning: Avoid contact with skin by wearing protective gloves when handling the inserts. Not for use in humans. Keep out of reach of children.
Environmental Warning: Store used (removed) EAZI-BREED CIDR Cattle Inserts in a plastic bag or other sealable container until they can be properly disposed in accordance with applicable local, state and Federal regulations. -
DO NOT USE
• An insert more than once. To prevent the potential transmission of venereal and blood born disease the EAZI-BREED CIDR Cattle Insert should be disposed after a single use.
• In beef or dairy heifers of insufficient size or age for breeding or in cattle with abnormal, immature or infected genital tracts.
• In beef cows that are less than 20 days postpartum for synchronization of estrus or advancement of first postpartum estrus because safety and effectiveness have not been evaluated.
• In lactating dairy cows less than 40 days postpartum for synchronization of estrus or synchronization of the return to estrus because safety and effectiveness have not been evaluated.
• In anestrous lactating dairy cows less than 42 days or greater than 78 days postpartum for induction of estrous cycles because safety and effectiveness have not been evaluated -
YOU MAY NOTICE
• Increased loss of EAZI-BREED CIDR Cattle Inserts in animals housed under crowded conditions, especially in heifers. Avoid crowded conditions during treatment as other cattle, particularly heifers, may remove EAZI-BREED CIDR Cattle Inserts by pulling on the tail of the EAZI-BREED CIDR Cattle Insert. If loss rates are high re-evaluate insertion technique and cattle handling facilities.
• Clear, cloudy, yellow or bloody mucus on the outside of EAZI-BREED CIDR Cattle Insert when removed from animals. The mucus may have an offensive odor. This is a result of irritation to the vaginal lining by the presence of the EAZI-BREED CIDR Cattle Insert, and generally clears between the time of removal and insemination. Such irritation does not affect fertility at inseminations following treatment.
• Use of EAZI-BREED Cattle Insert for periods of longer than 7 days may result in reduced fertility.
• Reduced conception rates to inseminations conducted immediately following removal of the EAZI-BREED CIDR Cattle Insert when used for induction of estrous cycles in anestrous lactating dairy cows. Such reductions in conception rate are not expected to result in reduced pregnancy rates.
• Reduced pregnancy rates to inseminations conducted immediately prior to administration of EAZI-BREED CIDR Cattle Inserts used for synchronizing the return to estrus in lactating -
DIRECTIONS
Lactating Dairy Cows
For Synchronization of Estrus in Lactating Dairy Cows:
• Administer one EAZI-BREED CIDR Cattle Insert per animal and remove 7 days later (for example if administered on a Monday remove the following Monday).
• Administer 5 mL LUTALYSE® Sterile Solution at the time of removal of the EAZI-BREED CIDR Cattle Insert.
• Observe animals for signs of estrus on Days 2 to 5 after removal of the EAZI-BREED CIDR Cattle Insert and inseminate animals found in estrus following typical herd practices.For Induction of Estrous Cycles in Anestrous Lactating Dairy Cows:
• For induction of estrous cycles in anestrous lactating dairy cows, anestrous dairy cows can be identified using any of the following methods:
- Cows not observed in estrus since calving.
- Cows diagnosed twice without a corpus luteum on either ovary via ultrasonography at a 7 to 14 day interval, such as on Day 35 and Day 42 post calving.
- Cows with low concentration of progesterone in two blood or milk samples collected at a 7 to 14 day interval, such as samples collected on Day 35 and 42 post calving.
• Administer one EAZI-BREED CIDR Cattle Insert per anestrous cow and remove 7 days later (for example if administered on a Monday remove the following Monday).
• If insemination is intended, observe cows on Days 2 to 5 after removal of the EAZI-BREED CIDR Cattle Insert and inseminate animals found in estrus following typical herd practices.For Synchronization of the Return to Estrus in Lactating Dairy Cows Inseminated at the Immediately Preceding Estrus:
• Administer one EAZI-BREED CIDR Cattle Insert per animal 14±1 days after insemination. Remove EAZI-BREED CIDR Cattle Insert 7 days later (for example, if administered on a Monday
remove on the following Monday).
• Observe animals for signs of estrus on Days 1 to 3 after removal of the EAZI-BREED CIDR Cattle Insert and inseminate animals about 12 hours after onset of estrus.
• Note: Do not administer LUTALYSE® Sterile Solution or other prostaglandin products to cows for synchronization of the return to estrus, as this will interrupt pregnancy that may have occurred at the immediately previous insemination.Suckled Beef Cows, Replacement Beef and Dairy Heifers: For synchronization of estrus in suckled beef cows and replacement beef and dairy heifers, advancement of first postpartum estrus in suckled beef cows, and advancement of first pubertal estrus in beef heifers:
• Administer one EAZI-BREED CIDR Cattle Insert per animal for 7 days (for example, if administered on a Monday remove on the following Monday).
• Inject 5 mL LUTALYSE® Sterile Solution (equivalent to 5 mg/mL dinoprost) 1 day prior to EAZI-BREED CIDR Cattle Insert removal, on Day 6 of the 7 day administration period.
• Observe animals for signs of estrus on Days 1 to 3 after removal of the EAZI-BREED CIDR Cattle Insert and inseminate animals about 12 hours after onset of estrus.Insertion:
1. Avoid contact with skin by wearing protective gloves when handling inserts.
2. Only use the specially designed EAZI-BREED CIDR Cattle Insert Applicator for administration.
3. Restrain cattle appropriately (head catch, squeeze chute, gate, etc.) prior to administration.
4. Wash the EAZI-BREED CIDR Cattle Insert Applicator in a non-irritating antiseptic solution, and then lubricate the front portion of the EAZI-BREED CIDR Cattle Insert Applicator with a veterinary obstetrical lubricant.
5. Push the flexible tail end of the EAZI-BREED CIDR Cattle Insert into the EAZI-BREED CIDR Cattle Insert Applicator taking care to assure the tail is extending upward through the slot of the EAZI-BREED CIDR Cattle Insert Applicator and is pointed toward the handle.
6. Fold the wings of the EAZI-BREED CIDR Cattle Insert to make it longer and continue to advance the EAZI-BREED CIDR Cattle Insert into the applicator until it is fully seated. When fully seated only the tips of the wings should protrude (one half inch) from the end of the EAZI-BREED CIDR Cattle Insert Applicator (see Figure 1 below).
7. Lubricate the protruding tips of the wings of the EAZI-BREED CIDR Cattle Insert with veterinary obstetrical lubricant.
8. Lift the tail of the animal and clean the exterior of the vulva.
9. Open the lips of the vulva and gently place the loaded EAZI-BREED CIDR Cattle Insert Applicator through the vulva. The slot in the EAZI-BREED CIDR Cattle Insert Applicator should face upwards (see Figure 2 below).
10. Once the loaded EAZI-BREED CIDR Cattle Insert Applicator is past the vulva slope the EAZI-BREED CIDR Cattle Insert Applicator slightly upwards (35-45° angle) by lowering the handle, and then forward, without forcing, until the EAZI-BREED CIDR Cattle Insert Applicator is fully inserted or resistance is felt (see Figure 3 below).
11. Squeeze the finger grips within the handle of the EAZI-BREED CIDR Cattle Insert Applicator to deposit the EAZI-BREED CIDR Cattle Insert in the anterior vagina (see Figure 4 below) and then pull the EAZI-BREED CIDR Cattle Insert Applicator backwards to remove it from the vagina.
12. With the EAZI-BREED CIDR Cattle Insert correctly placed, with the wings open in the anterior portion of the vagina, the tail of the EAZI-BREED CIDR Cattle Insert should be visible, pointing downward from the vulva of the animal. Tails of EAZI-BREED CIDR Cattle Inserts that protrude more than 2.5 inches from the vulva may be clipped to minimize removal by other animals.Removal:
1. Remove EAZI-BREED CIDR Cattle Inserts by pulling, gently but firmly, on the protruding polyester tail.
2. EAZI-BREED CIDR Cattle Inserts have been reported to reverse direction within the vagina; therefore, if the polyester tail of the insert is not visible on the day of removal, check the vagina to determine if an insert is present.
3. Used (removed) EAZI-BREED CIDR Cattle Inserts still contain some progesterone. Used EAZI-BREED CIDR Cattle Inserts must be stored in a sealable container until disposed. Sealed bag/container with used EAZI-BREED CIDR Cattle Inserts must be properly disposed in accordance with applicable local, state and Federal regulations.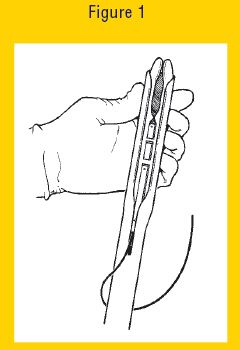
Figure 1 Eazi-Breed CIDR
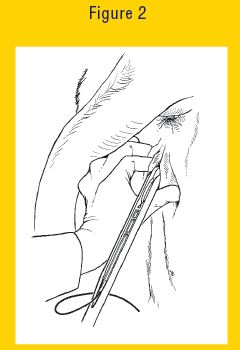
Figure 2 Eazi Breed CIDR
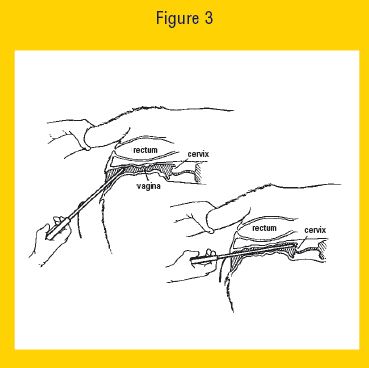
Figure 3 Eazi Breed CIDR
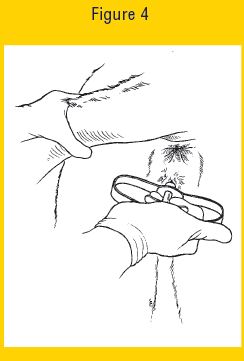
Figure 4 Eazi Breed CIDR
- OTHER INFORMATION
-
SPL UNCLASSIFIED SECTION
To report suspected adverse events, for technical assistance or to obtain a copy of the SDS, contact Zoetis at 1-888-963-8471. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888 -FDA-VETS or http://www.fda.gov/reportanimalae.
Restricted Drug (California) - use only as directed
Distributed by: Zoetis Inc.
Kalamazoo, MI 49007
39006604-04/19 - Inactive Ingredients
- PRINCIPAL DISPLAY PANEL - 10 Insert Bag Label
-
INGREDIENTS AND APPEARANCE
EAZI-BREED CIDR CATTLE INSERT
progesterone insert, extended releaseProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:54771-5207 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROGESTERONE (UNII: 4G7DS2Q64Y) (PROGESTERONE - UNII:4G7DS2Q64Y) PROGESTERONE 1.38 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-5207-1 10 in 1 BAG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141200 05/02/2002 Labeler - Zoetis Inc. (828851555)


