Label: FLORIVA PLUS- vitamin a palmitate, ascorbic acid, cholecalciferol, .alpha.-tocopherol succinate, d-, thiamine hydrochloride, riboflavin 5-phosphate sodium, niacinamide, pyridoxine hydrochloride, levomefolate glucosamine, cyanocobalamin, and sodium fluoride solution/ drops
- NHRIC Code(s): 52796-170-50
- Packager: BonGeo Pharmaceuticals, Inc.
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated April 4, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
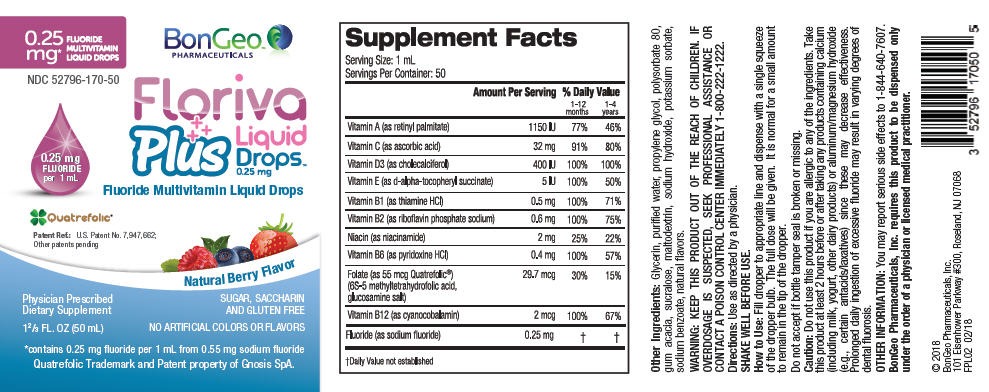
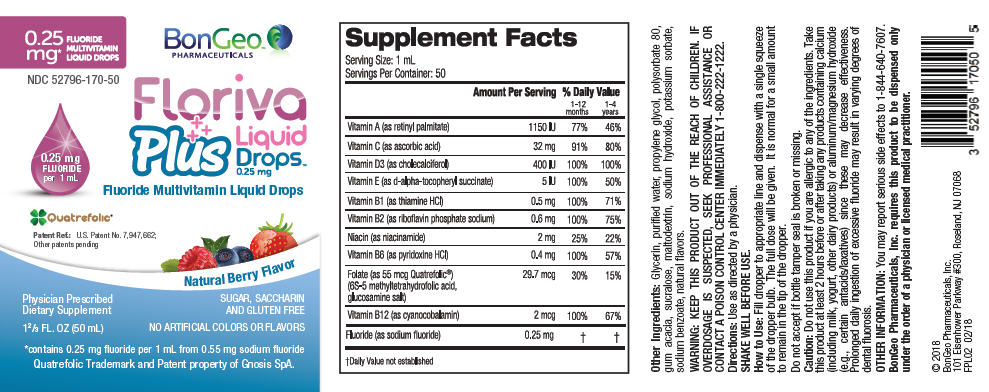
Supplement Facts Dosage Size: 1 mL Servings Per Container: 50 Amount per 1 mL Ingredient Amount Per Serving % Daily Value 1-12 months 1-4 years - *
- Daily Value not established.
Vitamin A (as retinyl palmitate) 345 mcg RAE 69% 115% Vitamin C (as ascorbic acid) 32 mg 64% 213% Vitamin D3 (as cholecalciferol) 10 mcg (400 IU) 100% 67% Vitamin E (as d-alpha-tocopheryl succinate) 3.4 mg (5 IU) 68% 57% Vitamin B1 (as thiamine HCl) 0.5 mg 167% 100% Vitamin B2 (as riboflavin phosphate sodium) 0.6 mg 150% 120% Niacin (as niacinamide) 2 mg 50% 33% Vitamin B6 (as pyridoxine HCl) 0.4 mg 133% 80% Folate (as 55 mcg Quatrefolic 49.5 mcg DFE 62% 33% (6S-5 methyl tetrahydrofolic acid, (29.7 mcg Folic Acid) glucosamine salt) Vitamin B12 (as cyanocobalamin) 2 mcg 400% 222% Fluoride (as sodium fluoride) 0.25 mg * * Other Ingredients: Glycerin, purified water, propylene glycol, polysorbate 80, gum acacia, sucralose, maltodextrin, sodium hydroxide, potassium sorbate, sodium benzoate, natural flavors.
- WARNING
- Directions
- How to Use
- SAFE HANDLING WARNING
-
Caution
Do not use this product if you are allergic to any of the ingredients. Take this product at least 2 hours before or after taking any products containing calcium (including milk, yogurt, other dairy products) or aluminum/magnesium hydroxide (e.g., certain antacids/laxatives) since these may decrease effectiveness. Prolonged daily ingestion of excessive fluoride may result in varying degrees of dental fluorosis.
- OTHER INFORMATION
- HEALTH CLAIM
-
PRINCIPAL DISPLAY PANEL - 50 mL Container Label
0.25
mg*
FLUORIDE
MULTIVITAMIN
LIQUID DROPSBonGeo™
PHARMACEUTICALSNDC 52796-170-50
0.25 mg
FLUORIDE
per 1 mLFloriva
Plus
Liquid
Drops™
0.25 mgFluoride Multivitamin Liquid Drops
Quatrefolic®
Patent Ref.: U.S. Patent No. 7,947,662;
Other patents pendingNatural Berry Flavor
Physician Prescribed
Dietary Supplement1⅔ FL. OZ (50 mL)
SUGAR, SACCHARIN
AND GLUTEN FREENO ARTIFICIAL COLORS OR FLAVORS
*contains 0.25 mg fluoride per 1 mL from 0.55 mg sodium fluoride
Quatrefolic Trademark and Patent property of Gnosis SpA.
-
INGREDIENTS AND APPEARANCE
FLORIVA PLUS
vitamin a palmitate, ascorbic acid, cholecalciferol, .alpha.-tocopherol succinate, d-, thiamine hydrochloride, riboflavin 5-phosphate sodium, niacinamide, pyridoxine hydrochloride, levomefolate glucosamine, cyanocobalamin, and sodium fluoride solution/ dropsProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:52796-170 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 1150 [iU] in 1 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 32 mg in 1 mL CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 400 [iU] in 1 mL .ALPHA.-TOCOPHEROL SUCCINATE, D- (UNII: LU4B53JYVE) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL SUCCINATE, D- 5 [iU] in 1 mL THIAMINE HYDROCHLORIDE (UNII: M572600E5P) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE HYDROCHLORIDE 0.5 mg in 1 mL RIBOFLAVIN 5'-PHOSPHATE SODIUM (UNII: 20RD1DZH99) (FLAVIN MONONUCLEOTIDE - UNII:7N464URE7E) FLAVIN MONONUCLEOTIDE 0.6 mg in 1 mL NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 2 mg in 1 mL PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE HYDROCHLORIDE 0.4 mg in 1 mL LEVOMEFOLATE GLUCOSAMINE (UNII: Q65PL71Q1A) (LEVOMEFOLATE GLUCOSAMINE - UNII:Q65PL71Q1A) LEVOMEFOLATE GLUCOSAMINE 29.7 ug in 1 mL CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 2 ug in 1 mL SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 0.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ACACIA (UNII: 5C5403N26O) SUCRALOSE (UNII: 96K6UQ3ZD4) MALTODEXTRIN (UNII: 7CVR7L4A2D) SODIUM HYDROXIDE (UNII: 55X04QC32I) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:52796-170-50 50 mL in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 03/16/2018 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value flavor Labeler - BonGeo Pharmaceuticals, Inc. (964822022)