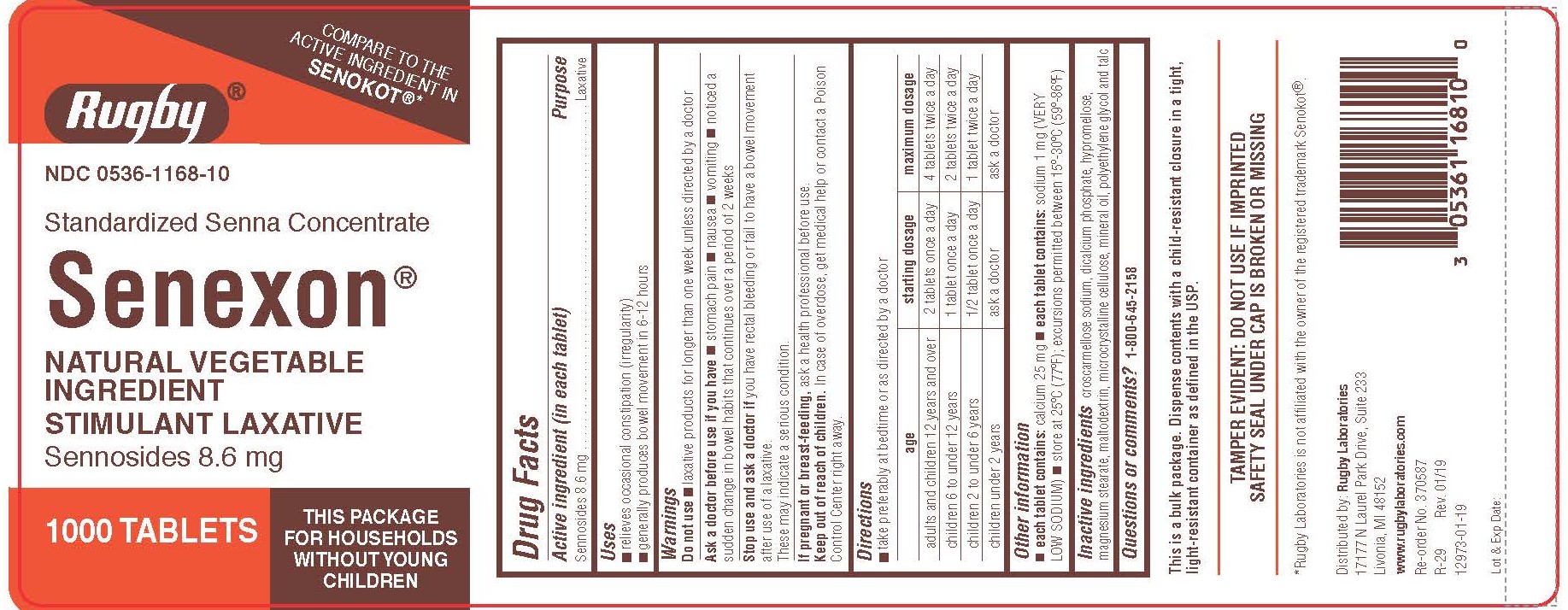
Label: SENNOSIDES tablet, film coated
- NDC Code(s): 0536-1168-01, 0536-1168-10
- Packager: Rugby Laboratories
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
- Uses
- WARNINGS
-
Directions
- take preferably at bedtime or as directed by a doctor
age starting dosage maximum dosage adults and children 12 years and over
2 tablets once a day
4 tablets twice a day
children 6 to under 12 years
1 tablet once a day
2 tablets twice a day
children 2 to under 6 years
1/2 tablet once a day
1 tablet twice a day
children under 2 years
ask a doctor
ask a doctor
- Other information
- INACTIVE INGREDIENT
- Questions or comments?
-
SPL UNCLASSIFIED SECTION
*Rugby Laboratories is not affiliated with the owner of the registered trademark Senokot®.
SAVE CARTON FOR COMPLETE DRUG FACTS
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Distributed by: Rugby Laboratories
17177 N Laurel Park Dr., Suite 233,
Livonia, MI 48152
www.rugbylaboratories.com
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SENNOSIDES
sennosides tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0536-1168 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES (UNII: 3FYP5M0IJX) (SENNOSIDES - UNII:3FYP5M0IJX) SENNOSIDES 8.6 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) LIGHT MINERAL OIL (UNII: N6K5787QVP) MAGNESIUM STEARATE (UNII: 70097M6I30) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSES (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color brown Score no score Shape ROUND Size 9mm Flavor Imprint Code 1122;1122 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0536-1168-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 02/14/2019 09/30/2025 2 NDC:0536-1168-01 1 in 1 CARTON 01/23/2019 11/30/2025 2 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 01/23/2019 11/30/2025 Labeler - Rugby Laboratories (079246066)