Label: STERILE WATER- water injection, solution
-
NDC Code(s):
0517-3005-01,
0517-3005-25,
0517-3010-01,
0517-3010-25, view more0517-3020-01, 0517-3020-25
- Packager: American Regent, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 31, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- BOXED WARNING (What is this?)
- DESCRIPTION:
-
PRECAUTIONS:
Unused amount should be discarded immediately following withdrawal of any portion of vial contents. Sterile Water for Injection is not isotonic and should not be injected directly into the body.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature).
Parenteral drug products should be inspected visually for particulate matter and discoloration, whenever solution and container permit.
-
HOW SUPPLIED:
Sterile Water for Injection, USP
NDC 0517-3005-25 5 mL Single Dose Vial Packaged in 25 NDC 0517-3010-25 10 mL Single Dose Vial Packaged in 25 NDC 0517-3020-25 20 mL Single Dose Vial Packaged in 25 NDC 0517-3050-25 50 mL Single Dose Vial Packaged in 25 AMERICAN
REGENT, INC.
SHIRLEY, NY 11967IN3005
Rev. 1/09 -
PRINCIPAL DISPLAY PANEL - 5 mL
Contain
NDC 0517-3005-01
STERILE WATER
FOR INJECTION, USP5 mL SINGLE DOSE VIAL
FOR DRUG DILUENT USE
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967

Carton
STERILE WATER
FOR INJECTION, USP
NDC 0517-3005-25
25 x 5 mL
SINGLE DOSE VIALSFOR DRUG DILUENT USE
Rx Only
No antimicrobial or other substance has been added. For use as a vehicle, solvent, or diluent for substances to be administered parenterally. Must be made approximately isotonic before intravenous use. Sterile, nonpyrogenic.
WARNING: DISCARD UNUSED PORTION.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature).
Directions for Use: See Package Insert.AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
Rev. 11/05

-
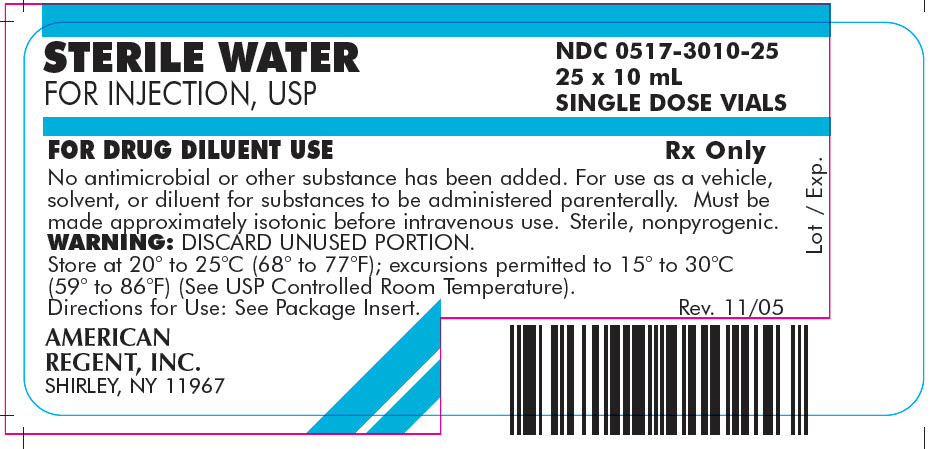
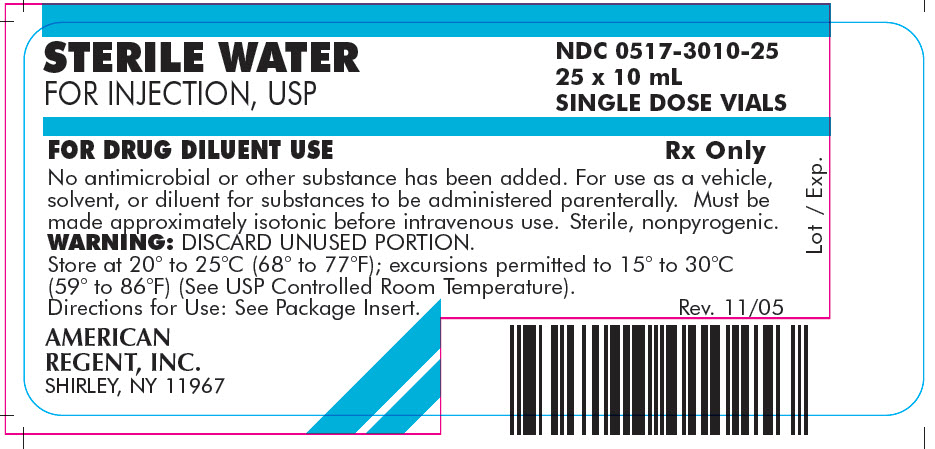
PRINCIPAL DISPLAY PANEL - 10 mL
Container
NDC 0517-3010-01
STERILE WATER
FOR INJECTION, USP10 mL SINGLE DOSE VIAL
FOR DRUG DILUENT USE
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967

Carton
STERILE WATER
FOR INJECTION, USP
NDC 0517-3010-25
25 x 10 mL
SINGLE DOSE VIALSFOR DRUG DILUENT USE
Rx Only
No antimicrobial or other substance has been added. For use as a vehicle, solvent, or diluent for substances to be administered parenterally. Must be made approximately isotonic before intravenous use. Sterile, nonpyrogenic.
WARNING: DISCARD UNUSED PORTION.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature).
Directions for Use: See Package Insert.AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
Rev. 11/05
-
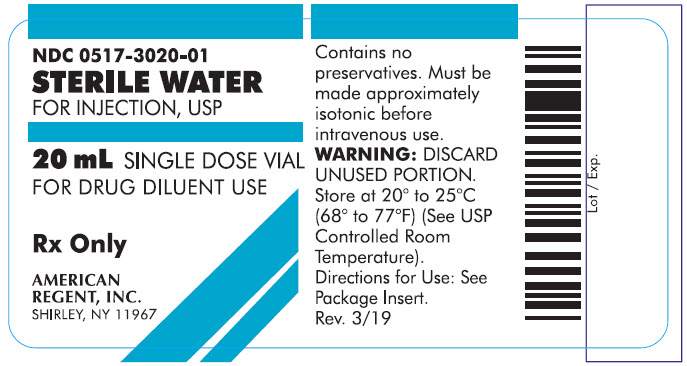
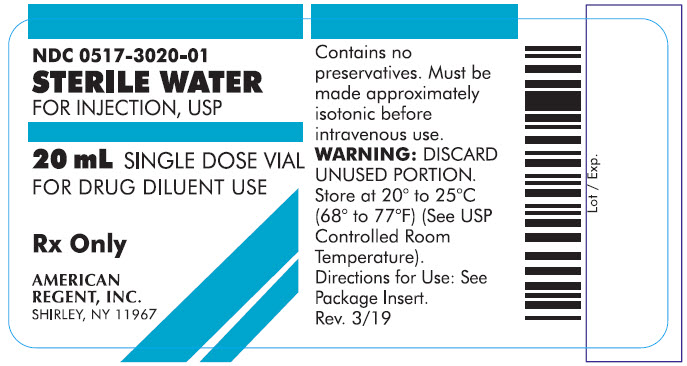
PRINCIPAL DISPLAY PANEL - 20 mL
Container
NDC 0517-3020-01
STERILE WATER
FOR INJECTION, USP20 mL SINGLE DOSE VIAL
FOR DRUG DILUENT USE
Rx Only
AMERICAN REGENT, INC.
SHIRLEY, NY 11967
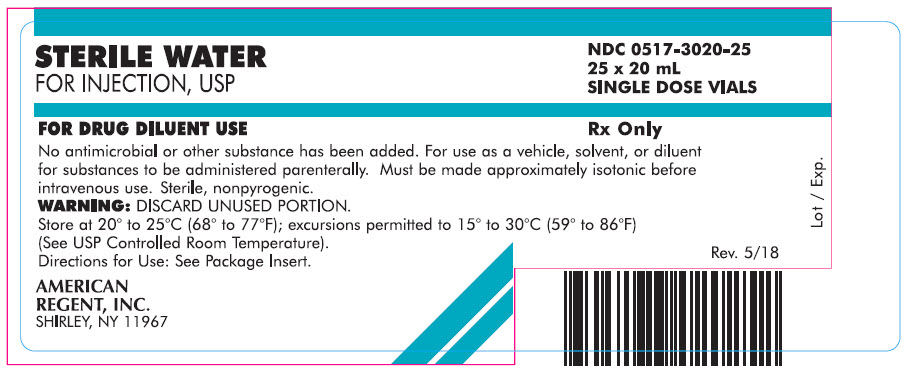
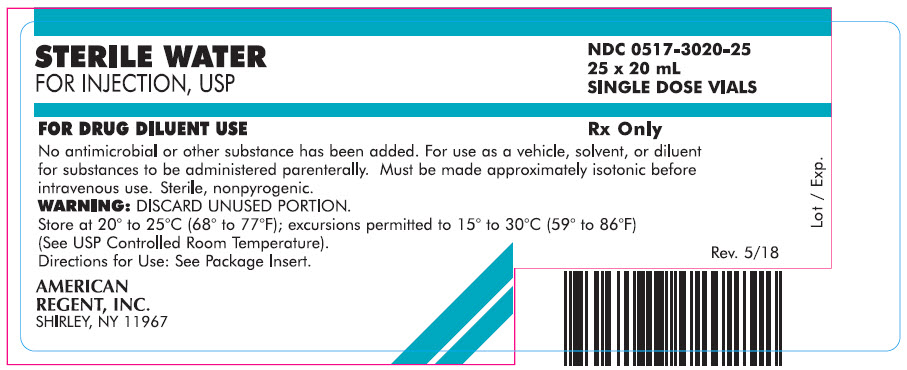
Carton
STERILE WATER
FOR INJECTION, USP
NDC 0517-3020-25
25 x 20 mL
SINGLE DOSE VIALSFOR DRUG DILUENT USE
Rx Only
No antimicrobial or other substance has been added. For use as a vehicle, solvent, or diluent for substances to be administered parenterally. Must be made approximately isotonic before intravenous use. Sterile, nonpyrogenic.
WARNING: DISCARD UNUSED PORTION.
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature).
Directions for Use: See Package Insert.AMERICAN
REGENT, INC.
SHIRLEY, NY 11967
Rev. 5/18
- Serialization Label - 5 mL
- Serialization Label - 10 mL
- Serialization Label - 20 mL
-
INGREDIENTS AND APPEARANCE
STERILE WATER
water injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0517-3005 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0517-3005-25 25 in 1 TRAY 09/30/1990 09/30/2021 1 NDC:0517-3005-01 5 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/30/1990 09/30/2021 STERILE WATER
water injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0517-3010 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0517-3010-25 25 in 1 TRAY 04/22/2022 1 NDC:0517-3010-01 10 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/22/2022 STERILE WATER
water injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0517-3020 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 1 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0517-3020-25 25 in 1 TRAY 04/22/2022 1 NDC:0517-3020-01 20 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/22/2022 Labeler - American Regent, Inc. (002033710) Establishment Name Address ID/FEI Business Operations American Regent, Inc. 002033710 analysis(0517-3005, 0517-3010, 0517-3020) , manufacture(0517-3005, 0517-3010, 0517-3020)