Label: VETRASEB CERADERM CM- chlorhexidine gluconate, miconazole nitrate shampoo
- NDC Code(s): 13985-447-08, 13985-447-16
- Packager: MWI/VETONE
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated April 9, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
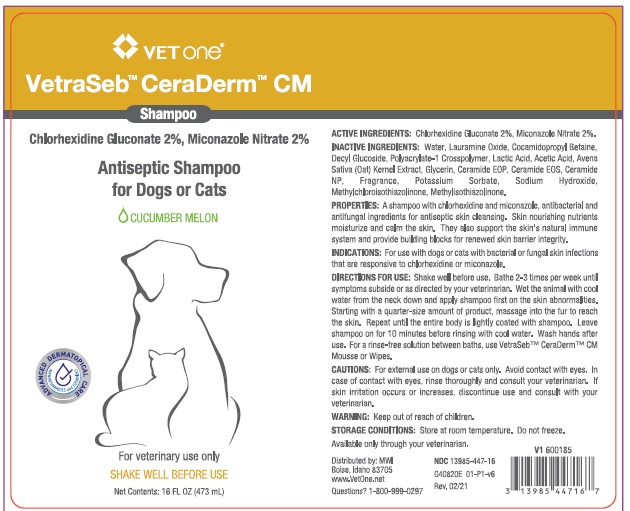
VetraSeb™ CeraDerm™ CM
Antiseptic Shampoo
for Dogs or Cats
CUCUMBER MELON
ACTIVE INGREDIENTS: Chlorhexidine Gluconate 2%, Miconazole Nitrate 2%.
INACTIVE INGREDIENTS: Water, Lauramine Oxide, Cocamidopropyl Betaine, Decyl Glucoside, Polyacrylate-1 Crosspolymer, Lactic Acid, Acetic Acid, Avena Sativa (Oat) Kernel Extract, Glycerin, Ceramide EOP, Ceramide EOS, Ceramide NP, Fragrance, Potassium Sorbate, Sodium Hydroxide, Methylchloroisothiazolinone, Methylisothiazoline.
PROPERTIES: A shampoo with chlorhexidine and miconazole, antibacterial and antifungal ingredients for skin cleansing. Skin nourishing nutrients moisturize and calm the skin. They also support the skin’s natural immune system and provide building blocks for renewed skin barrier integrity.
-
INDICATIONS & USAGE
INDICATIONS: For use with dogs or cats with bacterial or fungal skin infections that are responsive to chlorhexidine or miconazole.
DIRECTIONS FOR USE: Shake well before use. Bathe 2-3 times per week until symptoms subside or as directed by your veterinarian. Wet the animal with cool water from the neck down and apply shampoo first on the skin abnormalities. Starting with a quarter-size amount of product, massage into the fur to reach the skin. Repeat until the entire body is lightly coated with shampoo. Leave shampoo on for 10 minutes before rinsing with cool water. Wash hands after use. For a rinse-free solution between baths, use VetraSeb™ CeraDerm™ CM Mousse or Wipes.
- WARNINGS AND PRECAUTIONS
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 8 OUNCE (237 mL) Bottle
- PRINCIPAL DISPLAY PANEL - 16 OUNCE (473 mL) Bottle
-
INGREDIENTS AND APPEARANCE
VETRASEB CERADERM CM
chlorhexidine gluconate, miconazole nitrate shampooProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:13985-447 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 20 mg in 1 mL MICONAZOLE NITRATE (UNII: VW4H1CYW1K) (MICONAZOLE - UNII:7NNO0D7S5M) MICONAZOLE NITRATE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) DECYL GLUCOSIDE (UNII: Z17H97EA6Y) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) LACTIC ACID (UNII: 33X04XA5AT) ACETIC ACID (UNII: Q40Q9N063P) AVENANTHRAMIDES (UNII: 5PA41O4AIT) GLYCERIN (UNII: PDC6A3C0OX) CERAMIDE 1 (UNII: 5THT33P7X7) CERAMIDE EOS (UNII: CR0J8RN66K) CERAMIDE NP (UNII: 4370DF050B) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM HYDROXIDE (UNII: 55X04QC32I) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13985-447-08 237 mL in 1 BOTTLE, PLASTIC 2 NDC:13985-447-16 473 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/09/2021 Labeler - MWI/VETONE (019926120) Registrant - Ceva Sante Animale (261126049)