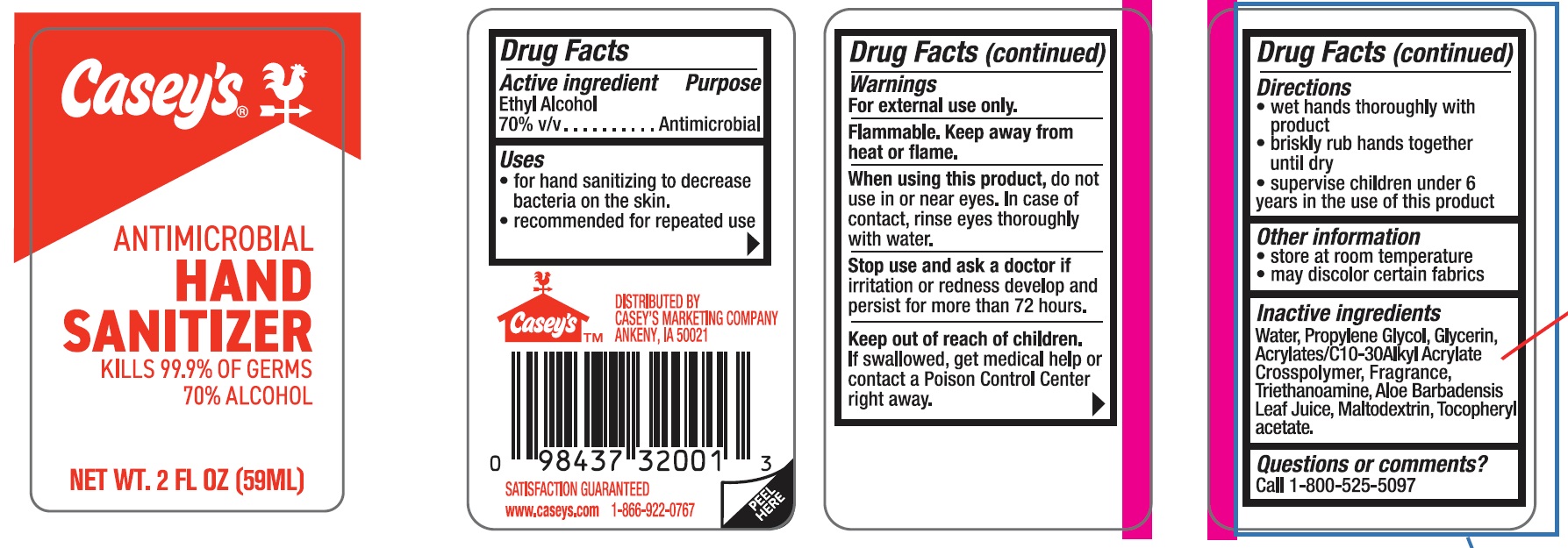
Label: CASEYS ANTIMICROBIAL HAND SANITIZER- ethyl alcohol gel
- NDC Code(s): 67751-071-01, 67751-071-02
- Packager: Navajo Manufacturing Company Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 29, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTActive ingredient - Ethyl Alcohol, 70% v/v
-
PURPOSEPurpose Antimicrobial
-
INDICATIONS & USAGEUses - for hand sanitizing to decrease bacteria on the skin - recommended for repeated use
-
WARNINGSWarnings - For external use only. Flammable, keep away from heat or flame.
-
WHEN USINGWhen using this product, do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water
-
STOP USEStop use and ask a doctor if irritation or redness develop and persist for more than 72 hours
-
KEEP OUT OF REACH OF CHILDRENKeep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
-
DOSAGE & ADMINISTRATIONDirections - wet hands thoroughly with product - briskly rub hands together until dry - supervise children under 6 years in the use of this product
-
STORAGE AND HANDLINGOther information - store at room temperature - may discolor certain fabrics
-
INACTIVE INGREDIENTInactive ingredients - Water (aqua), Propylene Glycol, Glycerin, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Fragrance, Triethanoamine, Aloe Barbadensis Leaf Juice, Maltodextrin ...
-
PRINCIPAL DISPLAY PANEL
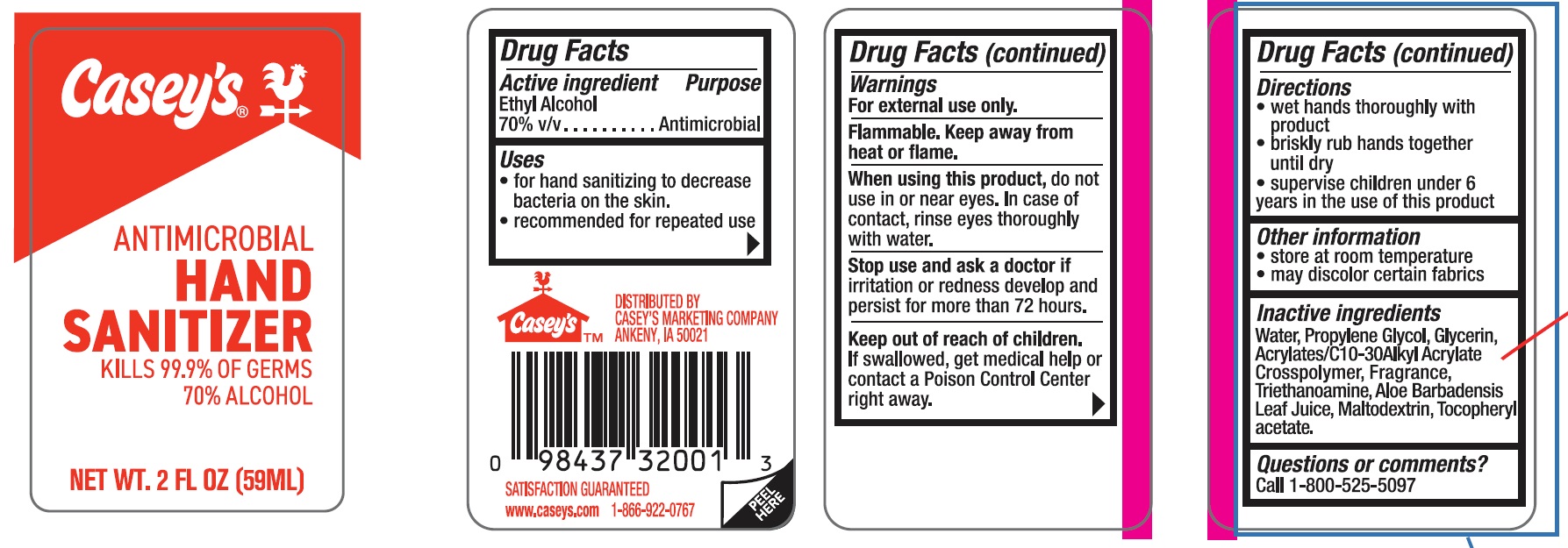
-
INGREDIENTS AND APPEARANCEProduct Information