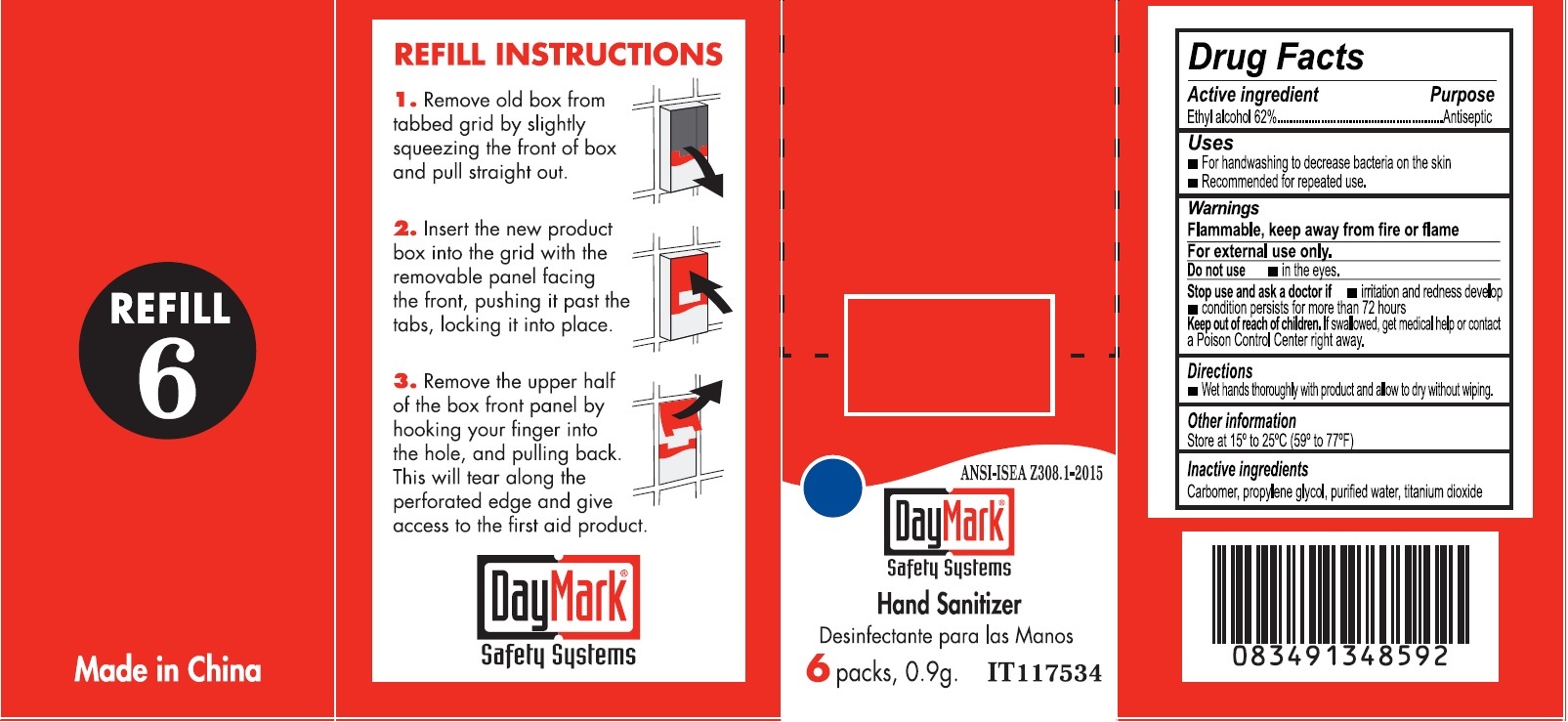
Label: REFILL 6- alcohol, water kit
- NDC Code(s): 49687-0010-1, 49687-0015-0, 49687-0019-0
- Packager: CMC Group, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts - Hand Sanitizer
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Drug Facts - Eye Wash
- Active ingredient
- Use
-
Warnings
For external use only.
When using this product
• to avoid contamination, do not touch tip of container to any surface • do not reuse • once opened, discard • obtain immediate medical treatment for all open wounds in or near the eyes
- Directions
- Other information
- Inactive ingredients
- Hand Sanitizer (49687-0015-0) Labeling:
- Eye Wash (50814-010-01) Labeling:
- Refill 6 (49687-0019-0) Labeling:
-
INGREDIENTS AND APPEARANCE
REFILL 6
alcohol, water kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49687-0019 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49687-0019-0 1 in 1 KIT 08/10/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 BOTTLE 5.4 g Part 2 1 TUBE 30 mL Part 1 of 2 HAND SANITIZER
alcohol gelProduct Information Item Code (Source) NDC:49687-0015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.62 g in 1 g Inactive Ingredients Ingredient Name Strength CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49687-0015-0 6 in 1 KIT 1 0.9 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/10/2016 Part 2 of 2 EYE WASH
water solutionProduct Information Item Code (Source) NDC:49687-0010 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 991 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49687-0010-1 1 in 1 BOX 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M018 08/10/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug part333 08/10/2016 Labeler - CMC Group, Inc. (117201448)