Label: MEIJER EXTRA STRENGTH COLD AND HOT THERAPYPAIN RELIEVING TOPICAL ANALGESIC- menthol, unspecified form and methyl salicylate cream
- NDC Code(s): 41250-378-01
- Packager: Meijer Distribution, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 21, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
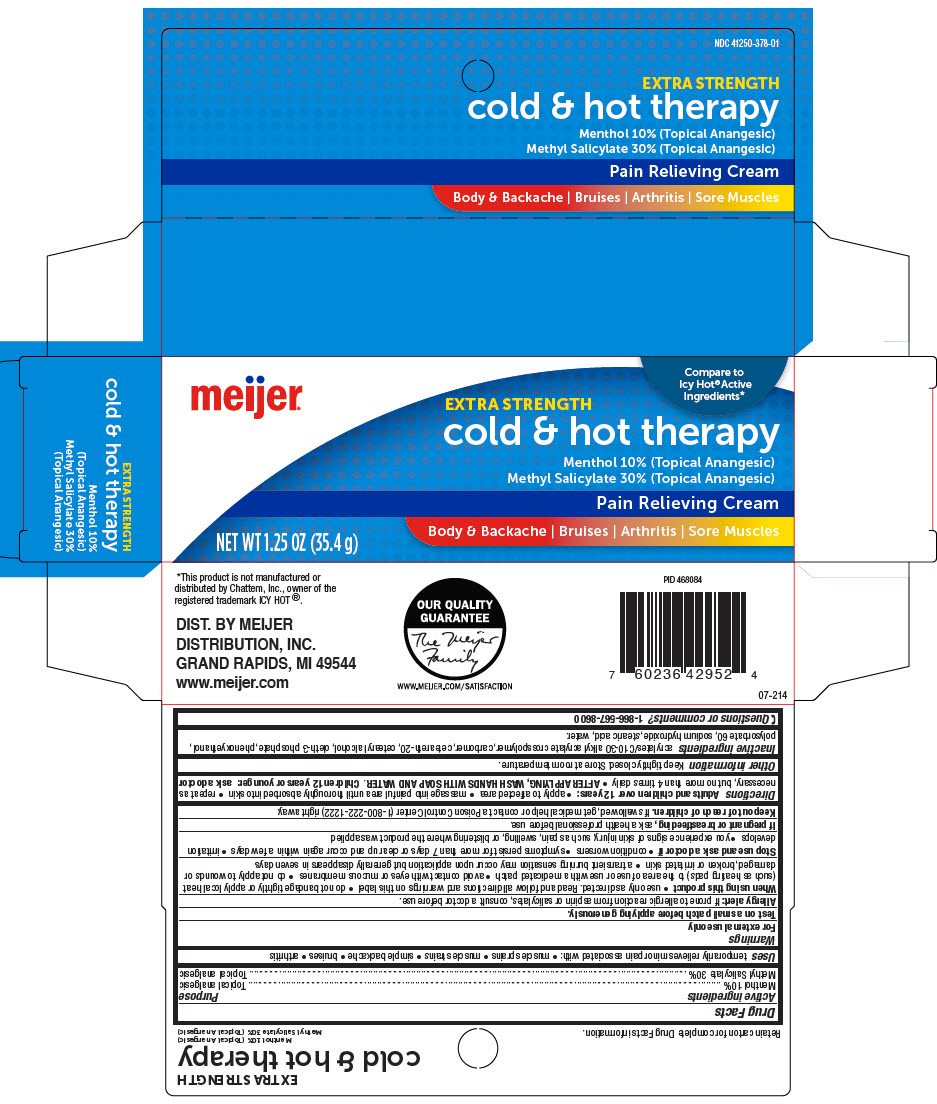
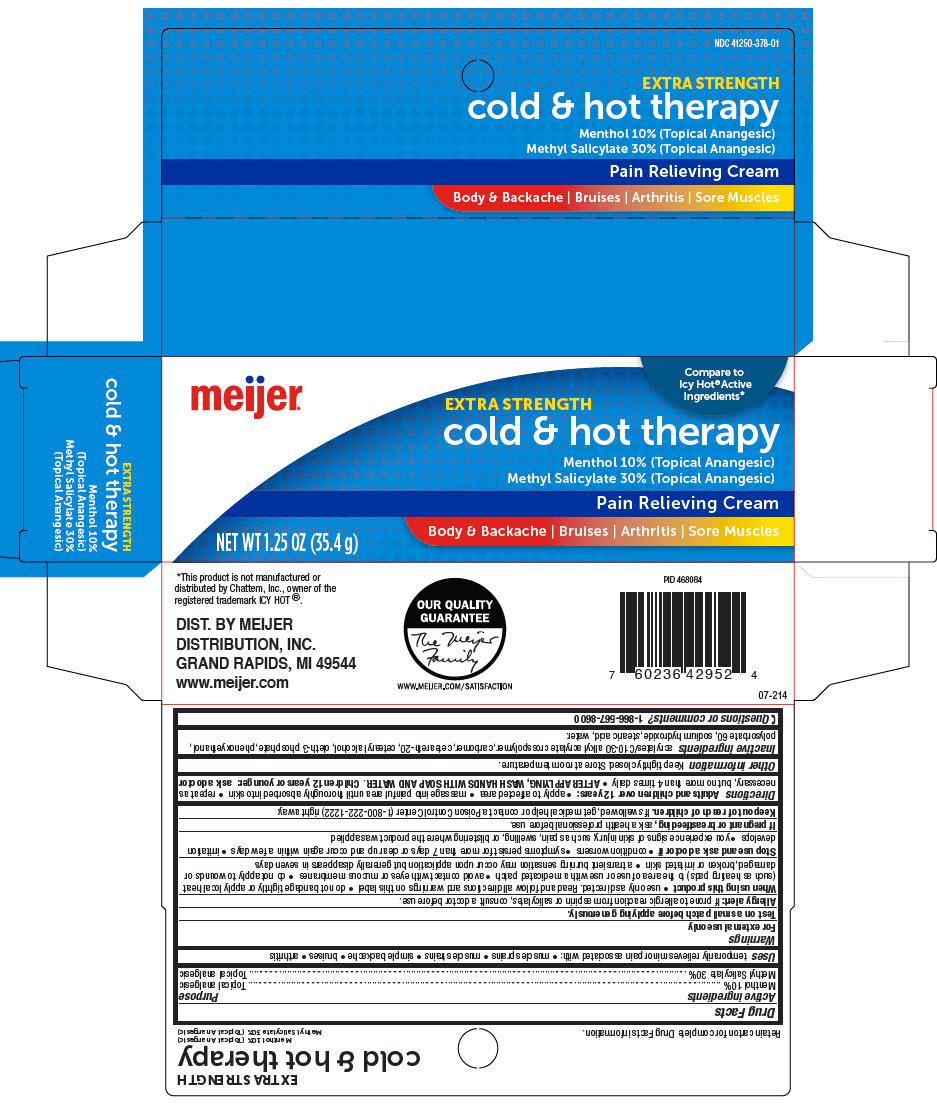
- ACTIVE INGREDIENT
- Uses
-
Warnings
For external use only
Test on a small patch before applying generously.
Allergy alert
If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
When using this product
- use only as directed. Read and follow all directions and warnings on this label
- do not bandage tightly or apply local heat (such as heating pads) to the area of use or use with a medicated patch
- avoid contact with eyes or mucous membranes
- do not apply to wounds or damaged, broken or irritated skin
- a transient burning sensation may occur upon application but generally disappears in seven days
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 35.4 g Tube Carton
-
INGREDIENTS AND APPEARANCE
MEIJER EXTRA STRENGTH COLD AND HOT THERAPYPAIN RELIEVING TOPICAL ANALGESIC
menthol, unspecified form and methyl salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41250-378 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Menthol, Unspecified Form (UNII: L7T10EIP3A) (Menthol, Unspecified Form - UNII:L7T10EIP3A) Menthol, Unspecified Form 100 mg in 1 g Methyl Salicylate (UNII: LAV5U5022Y) (Salicylic Acid - UNII:O414PZ4LPZ) Methyl Salicylate 300 mg in 1 g Inactive Ingredients Ingredient Name Strength Carbomer Interpolymer Type A (Allyl Sucrose Crosslinked) (UNII: 59TL3WG5CO) Carbomer Homopolymer, Unspecified Type (UNII: 0A5MM307FC) Polyoxyl 20 Cetostearyl Ether (UNII: YRC528SWUY) Cetostearyl Alcohol (UNII: 2DMT128M1S) Oleth-3 Phosphate (UNII: 8Q0Z18J1VL) Phenoxyethanol (UNII: HIE492ZZ3T) Polysorbate 60 (UNII: CAL22UVI4M) Sodium Hydroxide (UNII: 55X04QC32I) Stearic Acid (UNII: 4ELV7Z65AP) Water (UNII: 059QF0KO0R) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41250-378-01 1 in 1 CARTON 11/30/2020 1 35.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part348 11/30/2020 Labeler - Meijer Distribution, INC (006959555) Registrant - Garcoa, Inc. (036464697) Establishment Name Address ID/FEI Business Operations Sigan Industries INC. 255106239 MANUFACTURE(41250-378)