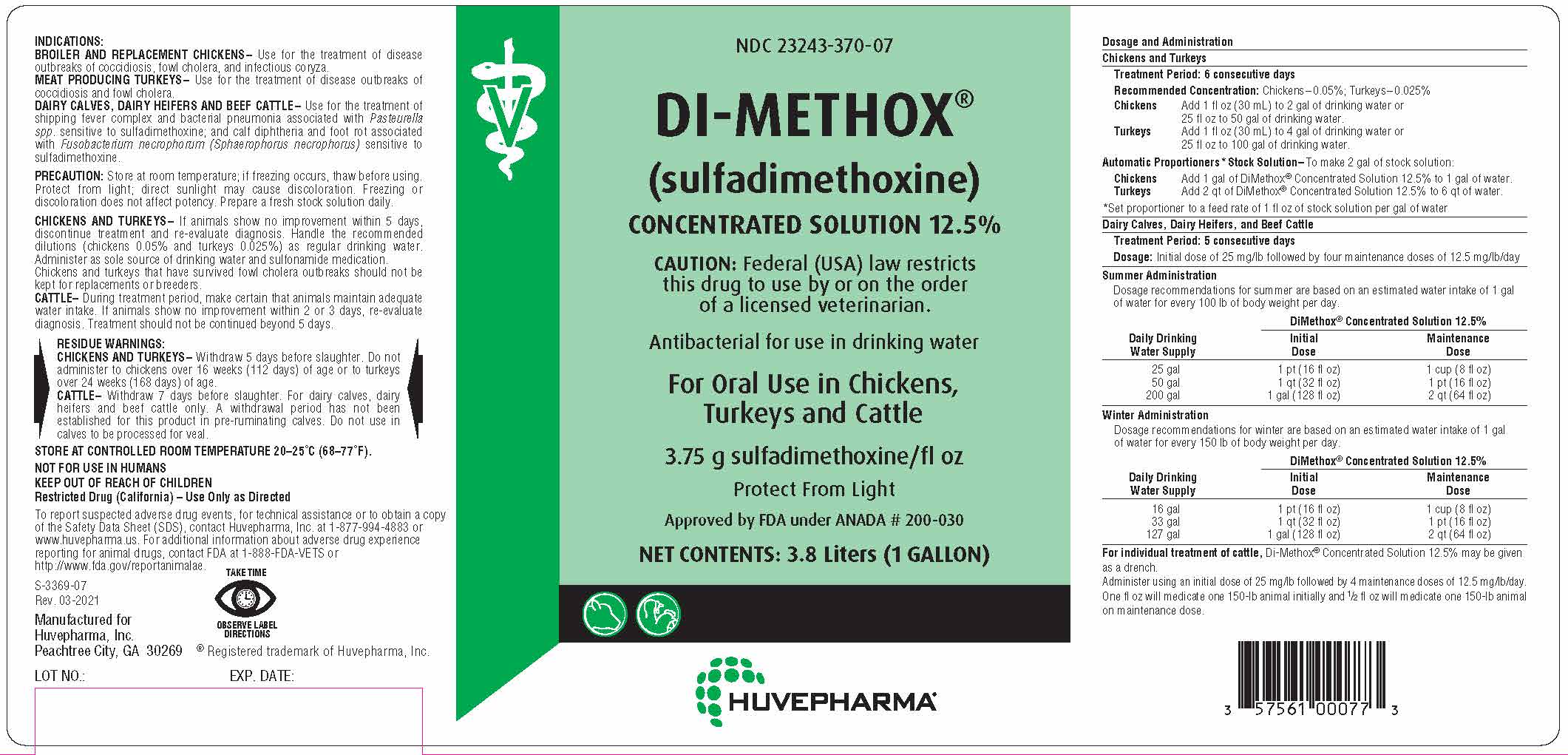
Label: DI-METHOX CONCENTRATED SOLUTION 12.5%- sulfadimethoxine concentrated solution 12.5% solution, concentrate
- NDC Code(s): 23243-3700-7
- Packager: Huvepharma, Inc
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated March 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- WARNINGS AND PRECAUTIONS
- SPL UNCLASSIFIED SECTION
-
VETERINARY INDICATIONS
INDICATIONS:
BROILER AND REPLACEMENT CHICKENS– Use for the treatment of disease
outbreaks of coccidiosis, fowl cholera, and infectious coryza.
MEAT PRODUCING TURKEYS– Use for the treatment of disease outbreaks of
coccidiosis and fowl cholera.
DAIRY CALVES, DAIRY HEIFERS AND BEEF CATTLE– Use for the treatment of
shipping fever complex and bacterial pneumonia associated with Pasteurella
spp. sensitive to sulfadimethoxine; and calf diphtheria and foot rot associated
with Fusobacterium necrophorum (Sphaerophorus necrophorus) sensitive to
sulfadimethoxine. -
PRECAUTIONS
PRECAUTION: Store at room temperature; if freezing occurs, thaw before using.
Protect from light; direct sunlight may cause discoloration. Freezing or
discoloration does not affect potency. Prepare a fresh stock solution daily.CHICKENS AND TURKEYS– If animals show no improvement within 5 days,
discontinue treatment and re-evaluate diagnosis. Handle the recommended
dilutions (chickens 0.05% and turkeys 0.025%) as regular drinking water.
Administer as sole source of drinking water and sulfonamide medication.
Chickens and turkeys that have survived fowl cholera outbreaks should not be
kept for replacements or breeders.CATTLE– During treatment period, make certain that animals maintain adequate
water intake. If animals show no improvement within 2 or 3 days, re-evaluate
diagnosis. Treatment should not be continued beyond 5 days. -
RESIDUE WARNING
RESIDUE WARNINGS:
CHICKENS AND TURKEYS– Withdraw 5 days before slaughter. Do not
administer to chickens over 16 weeks (112 days) of age or to turkeys
over 24 weeks (168 days) of age.
CATTLE– Withdraw 7 days before slaughter. For dairy calves, dairy
heifers and beef cattle only. A withdrawal period has not been
established for this product in pre-ruminating calves. Do not use in
calves to be processed for veal. - STORAGE AND HANDLING
-
USER SAFETY WARNINGS
NOT FOR USE IN HUMANS
KEEP OUT OF REACH OF CHILDREN
Restricted Drug (California) – Use Only as DirectedTo report suspected adverse drug events, for technical assistance or to obtain a copy
of the Safety Data Sheet (SDS), contact Huvepharma, Inc. at 1-877-994-4883 or
www.huvepharma.us. For additional information about adverse drug experience
reporting for animal drugs, contact FDA at 1-888-FDA-VETS or
http://www.fda.gov/reportanimalae. - SPL UNCLASSIFIED SECTION
-
DOSAGE & ADMINISTRATION
Dosage and Administration
Chickens and Turkeys
Treatment Period: 6 consecutive days
Recommended Concentration: Chickens–0.05%; Turkeys–0.025%
Chickens
Add 1 fl oz (30 mL) to 2 gal of drinking water or
25 fl oz to 50 gal of drinking water.
Turkeys
Add 1 fl oz (30 mL) to 4 gal of drinking water or
25 fl oz to 100 gal of drinking water.
Automatic Proportioners*Stock Solution–To make 2 gal of stock solution:
Chickens
Add 1 gal of DiMethox® Concentrated Solution 12.5% to 1 gal of water.
Turkeys
Add 2 qt of DiMethox® Concentrated Solution 12.5% to 6 qt of water.
*Set proportioner to a feed rate of 1 fl oz of stock solution per gal of water
Dairy Calves, Dairy Heifers, and Beef Cattle
Treatment Period: 5 consecutive days
Dosage: Initial dose of 25 mg/lb followed by four maintenance doses of 12.5 mg/lb/day
Summer Administration
Dosage recommendations for summer are based on an estimated water intake of 1 gal
of water for every 100 lb of body weight per day.DiMethox® Concentrated Solution 12.5%
Daily Drinking
Water Supply
Initial
Dose
Maintenance
Dose
25 gal
1 pt (16 fl oz)
1 cup (8 fl oz)
50 gal
1 qt (32 fl oz)
1 pt (16 fl oz)
200 gal
1 gal (128 fl oz)
2 qt (64 fl oz)
Winter Administration
Dosage recommendations for winter are based on an estimated water intake of 1 gal
of water for every 150 lb of body weight per day.DiMethox® Concentrated Solution 12.5%
Daily Drinking
Water Supply
Initial
Dose
Maintenance
Dose
16 gal
1 pt (16 fl oz)
1 cup (8 fl oz)
33 gal
1 qt (32 fl oz)
1 pt (16 fl oz)
127 gal
1 gal (128 fl oz)
2 qt (64 fl oz)
For individual treatment of cattle, Di-Methox® Concentrated Solution 12.5% may be given
as a drench.
Administer using an initial dose of 25 mg/lb followed by 4 maintenance doses of 12.5 mg/lb/day.
One fl oz will medicate one 150-lb animal initially and 1/2 fl oz will medicate one 150-lb animal
on maintenance dose. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DI-METHOX CONCENTRATED SOLUTION 12.5%
sulfadimethoxine concentrated solution 12.5% solution, concentrateProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:23243-3700 Route of Administration Oral Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sulfadimethoxine (UNII: 30CPC5LDEX) (SULFADIMETHOXINE - UNII:30CPC5LDEX) Sulfadimethoxine 3750 mg in 0.2366 L Inactive Ingredients Ingredient Name Strength SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM METABISULFITE (UNII: 4VON5FNS3C) EDETATE DISODIUM (UNII: 7FLD91C86K) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:23243-3700-7 3.8 L in 1 JUG Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200030 02/01/2009 Labeler - Huvepharma, Inc (619153559) Registrant - Huvepharma EOOD (552671651) Establishment Name Address ID/FEI Business Operations Sparhawk Laboratories, Inc. 147979082 manufacture, analysis