Label: ROBITUSSIN HONEY SEVERE COUGH, FLU PLUS SORE THROAT- acetaminophen, dextromethorphan hbr solution
- NDC Code(s): 0031-8771-12, 0031-8771-18
- Packager: Haleon US Holdings LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated April 26, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each 20 mL)
- Purposes
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Liver warning
This product contains acetaminophen.
Severe liver damage may occur if you take
- •
- more than 6 doses in any 24-hour period, which is the maximum daily amount
- •
- with other drugs containing acetaminophen
- •
- 3 or more alcoholic drinks every day while using this product
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- •
- skin reddening
- •
- blisters
- •
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Ask a doctor before use if you have
- •
- liver disease
- •
- cough that occurs with too much phlegm (mucus)
- •
- a breathing problem or chronic cough that lasts or as occurs with smoking, asthma, or emphysema
Ask a doctor or pharmacist before use if you are
- •
- taking the blood thinning drug warfarin
- •
- taking any other pain reliever/fever reducer
-
DOSAGE & ADMINISTRATION
Directions
- •
- do not take more than 6 doses in any 24-hour period
- •
- do not exceed recommended dosage. Taking more than the recommended dose (overdose) may cause serious liver damage.
- •
- measure only with dosing cup provided
- •
- keep dosing cup with product
- •
- mL = milliliter
- •
- this adult product is not intended for use in children under 12 years of age
age dose adults and children
12 years and over
20 mL every 4 hours
children under 12 years
do not use
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
-
Additional information
Packaged with Tamper-Evident bottle cap. Do Not Use if breakable ring is separated or missing.
PARENTS:
Learn about teen medicine abuse
www.StopMedicineAbuse.org
Distributed by: Haleon, Warren, NJ 07059
©2024 Haleon group of companies or its licensor.
Trademarks are owned by or licensed to the Haleon group of companies.
For most recent product information, visit www.robitussin.com
Pat. Info www.productpats.com
Made in Canada
-
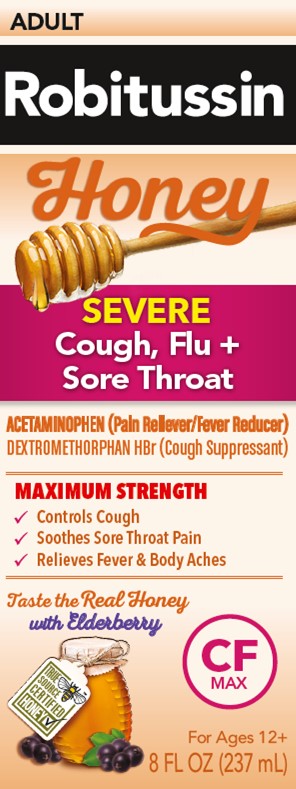
PRINCIPAL DISPLAY PANEL
Robitussin
Honey
ADULT
SEVERE
Cough, Flu +
Sore ThroatACETAMINOPHEN (Pain Reliever/Fever Reducer)
DEXTROMETHORPHAN HBr (Cough Suppressant)NON-DROWSY
MAXIMUM STRENGTH
- ✓
- Controls Cough
- ✓
- Soothes Sore Throat Pain
- ✓
- Relieves Fever & Body Aches
Taste the Real Honey
with ElderberryCF
MAXFor Ages 12+
8 FL OZ (237 mL)
62000000207916 – Front Carton
-
INGREDIENTS AND APPEARANCE
ROBITUSSIN HONEY SEVERE COUGH, FLU PLUS SORE THROAT
acetaminophen, dextromethorphan hbr solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0031-8771 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 650 mg in 20 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg in 20 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) GLYCERIN (UNII: PDC6A3C0OX) HONEY (UNII: Y9H1V576FH) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SODIUM GLUCONATE (UNII: R6Q3791S76) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) ZINC GLUCONATE (UNII: U6WSN5SQ1Z) Product Characteristics Color ORANGE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0031-8771-12 1 in 1 CARTON 06/15/2020 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:0031-8771-18 1 in 1 CARTON 06/15/2020 2 237 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 06/15/2020 Labeler - Haleon US Holdings LLC (079944263)