Label: SODIUM SULFACETAMIDE liquid
- NDC Code(s): 42192-129-16
- Packager: Acella Pharmaceuticals, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 11, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
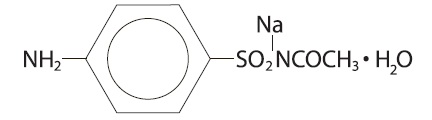
Sodium sulfacetamide is C 8H 9N 2NaO 3S·H 2O with molecular weight of 254.24. Chemically, it is Acetamide N-[(4-aminophenyl) sulfonyl]-acetamide, monosodium salt, monohydrate. The structural formula is:
Each gram of Sodium Sulfacetamide 10% Wash contains 100 mg of Sodium Sulfacetamide, USP in a formulation containing cocamidopropyl betaine, disodium EDTA,PEG-150 pentaerythrityl tetrastearate and PEG 6 caprylic/capric glycerides, glyceryl monostearate, methyl paraben, PEG-60 almond triglyceride, polysorbate 60, purified water, sodium lauryl sulfate and sodium thiosulfate.
Sodium Sulfacetamide is an odorless, white, crystalline powder with a bitter taste. It is freely soluble in water, sparingly soluble in alcohol, while practically insoluble in benzene, in chloroform and in ether.
-
CLINICAL PHARMACOLOGY:
Sodium sulfacetamide exerts a bacteriostatic effect against sulfonamide sensitive Gram-positive and Gram-negative microorganisms commonly isolated from secondary cutaneous pyogenic infections. It acts by restricting the synthesis of folic acid required by bacteria for growth, by its competition with para-aminobenzoic acid. There is no clinical data available on the degree and rate of systemic absorption of Sodium Sulfacetamide 10% Wash when applied to the skin or scalp. However, significant absorption of sodium sulfacetamide through the skin has been reported.
The following in vitro data is available but the clinical significance is not known. Organisms which show susceptibility to sodium sulfacetamide are: Streptococci, Staphylococci, E. coli, Klebsiella pneumonia, Pseudomonas pyocyanea, Salmonella species, Proteus vulgaris, Nocardia and Actinomyces .
- INDICATIONS:
- CONTRAINDICATIONS:
-
WARNINGS:
Sulfonamides are known to cause Stevens-Johnson syndrome in hypersensitivie individuals. Stevens-Johnson syndrome also has been reported following the use of sodium sulfacetamide topically. Cases of drug induced systemic lupus erythematosus from topical sulfacetamide also have been reported. In one of these cases, there was a fatal outcome. KEEP OUT OF THE REACH OF CHILDREN.
- PRECAUTIONS:
-
GENERAL PRECAUTIONS
General - Nonsusceptible organisms, including fungi, may proliferate with the use of this preparation. Hypersensitivity reactions may recur when a sulfonamide is readministered, irrespective of the route of administration, and cross hypersensitivity between different sulfonamides may occur. If Sodium Sulfacetamide 10% Wash produces signs of hypersensitivity or other untoward reactions, discontinue use of the preparation. Systemic absorption of topical sulfonamides is greater following application to large, infected, abraded, denuded, or severely burned areas. Under these circumstances, potentially any of the adverse effects produced by the systemic administration of these agents could occur and appropriate observations and laboratory determinations should be performed.
-
INFORMATION FOR PATIENTS
Information for patients - Patients should discontinue use of Sodium Sulfacetamide 10% Wash if the condition worsens, or is a rash develops in the area being treated or elsewhere. Sodium Sulfacetamide 10% Wash also should be discontinued promptly and the physician notified if any arthritis, fever or sores in the mouth develop.
- Drug Interactions:
-
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
Carcinogenesis, Mutagenesis and Impairment of Fertility - Long-term studies in animals have not been performed to evaluate carcinogenic potential. Studies on reproduction and fertility also have not been performed. Chromosomal nondisjunction in the yeast, Saccharomyces cerevisiae, following application of sodium sulfacetamide has been reported. The significance of this finding to the topical use of sodium sulfacetamide in the human is not known.
-
PREGNANCY:
Category C. Animal reproduction studies have not been conducted with Sodium Sulfacetamide 10% Wash. It is not known whether Sodium Sulfacetamide 10% Wash can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium Sulfacetamide 10% Wash should be given to a pregnant woman only if clearly needed or when potential benefits outweigh potential hazards to the fetus.
- NURSING MOTHERS:
- PEDIATRIC USE:
-
ADVERSE REACTIONS:
Reports of irritation and hypersensitivity to sodium sulfacetamide are uncommon. The following adverse reactions, reported after administration of sterile ophthalmic sodium sulfacetamide, are noteworthy: instances of Stevens-Johnson syndrome and instances of local hypersensitivity which progressed to a syndrome resembling systemic lupus erythematosus; in one case a fatal outcome was reported. (See warnings.)
-
OVERDOSAGE:
The oral LD 50 of sulfacetamide in mice is 16.5 g/kg. In the event of overdosage, emergency treatment should be started immediately. Manifestations: Overdosage may cause nausea and vomiting. Large oral overdosage may cause hematuria, crystalluria and renal shutdown due to the precipitation of sulfa crystals in the renal tubules and the urinary tract. For treatment, contact your local Poison Control Center.
-
DOSAGE AND ADMINISTRATION:
Seborrheic dermatitis including seborrhea sicca - Wash affected areas twice daily (morning and evening), or as directed by your physician. Avoid contact with eyes or mucous membranes. Wet skin and liberally apply to areas to be cleansed, massage gently into skin working into a full lather, rinse thoroughly and pat dry. Rinsing with plain water will remove any excess medication. Repeat application as described for eight to ten days. If skin dryness occurs it may be controlled by rinsing cleanser off sooner or using less frequently. Regular shampooing following Sodium Sulfacetamide 10% Wash is not necessary, but the hair should be shampooed at least once a week. As the condition subsides, the interval between applications may be lengthened. Applications once or twice weekly or every other week may prevent recurrence. Should the condition recur after stopping therapy, the application of Sodium Sulfacetamide 10% Wash should be reinstated as at the beginning of treatment.
Secondary Cutaneuos Bacterial Infections - Wet skin and liberally apply to areas to be cleansed, massage gently into skin working into a full lather, rinse thoroughly and pat dry. Rinsing with plain water will remove any excess medication. Repeat application as described for eight to ten days. if skin dryness occurs it may be controlled by rinsing cleanser off sooner or using less often.
- HOW SUPPLIED:
-
STORAGE AND HANDLING
Store at controlled room temperature, 15° - 30°C (59° - 86°F). Do not freeze.
Note: Store upright. Protect from freezing and excessive heat. The wash may tend to darken slightly on storage. Slight discoloration does not impair the efficacy or safety of the product. Occasionally, a slight yellowish discoloration may occur when an excessive amount of the wash is used and comes in contact with white fabrics. This discoloration, however, presents no problem, as it is readily removed by ordinary laundering without bleaches.
All prescription substitutions using this product shall be made subject to state and federal statutes as applicable. NOTE: this is not an Orange Book product and has not been subjected to FDA therapeutic equivalency or other equivalency testing. No representation is made as to generic status or bioequivalency. Each person recommending a prescription substitution using this product shall make such recommendations based on each such person’s professional opinion and knowledge, upon evaluating the active ingredients, excipients, inactive ingredients and chemical formulation information provided herein.
- SPL UNCLASSIFIED SECTION
- PACKAGE LABEL - 10% Wash
-
INGREDIENTS AND APPEARANCE
SODIUM SULFACETAMIDE
sodium sulfacetamide liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:42192-129 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFACETAMIDE SODIUM (UNII: 4NRT660KJQ) (SULFACETAMIDE - UNII:4965G3J0F5) SULFACETAMIDE SODIUM 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) EDETATE DISODIUM (UNII: 7FLD91C86K) PENTAERYTHRITYL TETRASTEARATE (UNII: W9Q3DZS0EG) CAPRYLIC/CAPRIC MONO/DIGLYCERIDES (UNII: U72Q2I8C85) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) METHYLPARABEN (UNII: A2I8C7HI9T) PEG-60 ALMOND GLYCERIDES (UNII: 4Y0E651N0F) POLYSORBATE 60 (UNII: CAL22UVI4M) WATER (UNII: 059QF0KO0R) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) SODIUM THIOSULFATE (UNII: HX1032V43M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42192-129-16 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/04/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/04/2011 Labeler - Acella Pharmaceuticals, LLC (825380939) Registrant - Acella Pharmaceuticals, LLC (825380939) Establishment Name Address ID/FEI Business Operations Acella Pharmaceuticals, LLC 825380939 manufacture(42192-129)


