Label: HYDROCORTISONE ACETATE suppository
- NDC Code(s): 75834-147-06, 75834-147-12, 75834-147-24
- Packager: Nivagen Pharmaceuticals, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
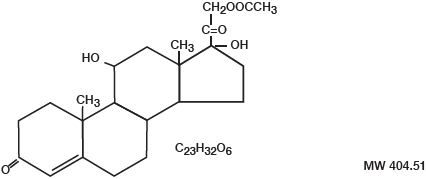
- DESCRIPTION
-
CLINICAL PHARMACOLOGY
In normal subjects, about 26% of hydrocortisone acetate is absorbed when the suppository is applied to the rectum. Absorption of hydrocortisone acetate may vary across abraded or inflamed surfaces. Topical steroids are primarily effective because of their anti-inflammatory, anti-pruritic and vasoconstrictive action.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
Do not use hydrocortisone acetate suppositories unless adequate proctologic examination is made.
If irritation develops, the product should be discontinued and appropriate therapy instituted.
In the presence of an infection, the use of an appropriate antifungal or antibacterial agent should be instituted. If a favorable response does not occur promptly, hydrocortisone acetate should be discontinued until the infection has been adequately controlled.
Carcinogenesis
No long term studies in animals have been performed to evaluate the carcinogenic potential of corticosteroid suppositories.
INFORMATION FOR PATIENTS
Staining of fabric may occur with use of the suppository. Precautionary measures are recommended.
PREGNANCY CATEGORY C
In laboratory animals, topical steroids have been associated with an increase in the incidence of fetal abnormalities when gestating females have been exposed to rather low dosage levels. There are no adequate and well controlled studies in pregnant women.
Hydrocortisone acetate suppositories should only be used during pregnancy if the potential benefit justifies the risk to the fetus. Drugs of this class should not be used extensively on pregnant patients, in large amounts, or for prolonged periods of time.
It is not known whether this drug is excreted in human milk and because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from hydrocortisone acetate suppositories, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
The following local adverse reactions have been reported with hydrocortisone acetate suppositories: burning, itching, irritation, dryness, folliculitis, hypopigmentation, allergic contact dermatitis, secondary infection.
To report SUSPECTED ADVERSE REACTIONS, contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
For rectal administration. Detach one suppository from strip of suppositories. Hold suppository upright. Separate tabs at top opening and pull downward from the pointed end. Continue pulling downward to almost the full length of the suppository. Carefully remove the suppository from the pocket. Avoid excessive handling of the suppository which is designed to melt at body temperature. Insert suppository into the rectum with gentle pressure, pointed end first. Insert one suppository in the rectum twice daily, morning and night for two weeks, in nonspecific proctitis. In more severe cases, one suppository three times a day or two suppositories twice daily. In factitial proctitis, the recommended duration of therapy is six to eight weeks or less, according to the response of the individual case.
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 25 mg Suppository Blister Pack Carton
-
INGREDIENTS AND APPEARANCE
HYDROCORTISONE ACETATE
hydrocortisone acetate suppositoryProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:75834-147 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 25 mg Inactive Ingredients Ingredient Name Strength HYDROGENATED PALM KERNEL OIL (UNII: FM8D1RE2VP) Product Characteristics Color WHITE Score no score Shape BULLET Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75834-147-12 2 in 1 CARTON 05/15/2020 1 NDC:75834-147-06 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:75834-147-24 4 in 1 CARTON 05/15/2020 2 NDC:75834-147-06 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug other 05/15/2020 Labeler - Nivagen Pharmaceuticals, Inc. (052032418)