Label: SPHERUSOL- coccidioides immitis spherule-derived skin test antigen injection, solution
- NDC Code(s): 59584-140-01
- Packager: Nielsen BioSciences, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 1, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Spherusol ® safely and effectively. See full prescribing information for Spherusol ®.
Coccidioides immitis Spherule-Derived Skin Test Antigen-
Spherusol ®
Solution for Intradermal Injection
Initial U.S. Approval: July 2011
Nielsen BioSciences, Inc. U.S. Lic. No. 1903
Mfd. by Allermed Laboratories, Inc. U.S. Lic. No. 0467WARNING
See full prescribing information for complete boxed warning
- The expected response to Spherusol ® is a local area of inflammation at the site of the skin test. The reaction is usually dime to quarter size reaching maximum diameter between 24 and 48 hours. Larger accelerated reactions can occur, which may require treatment with local cold compresses and anti-inflammatory medication. ( 2.3, 6.1)
- Systemic reactions can occur with skin test antigens and in certain individuals these reactions may be life-threatening or cause death. Emergency measures and personnel trained in their use should be immediately available. Patients should be observed for at least 20 minutes following the administration of a skin test. ( 5.3, 6.2)
- Spherusol ® should never be given intravenously. ( 5)
- To report SUSPECTED ADVERSE REACTIONS, contact Nielsen BioSciences, Inc. at (855) 855-1212 or MEDWATCH, Food and Drug Administration (FDA), 5600 Fishers Lane, Rockville, MD 20852-9782. Telephone: (800) 332-1088 or www.vaers.hhs.gov. ( 6.2)
INDICATIONS AND USAGE
- Spherusol ® is a skin test antigen indicated for the detection of delayed-type hypersensitivity to Coccidioides immitis in individuals with a history of pulmonary coccidioidomycosis. Spherusol ® is approved for use in individuals 18-64 years of age.
- The use of Spherusol ® to detect delayed-type hypersensitivity responses in a general population with unknown exposure to C. immitis has not been evaluated.
- Persons with acute or disseminated coccidioidomycosis may not develop a delayed-type hypersensitivity response to Spherusol ®.
- Persons with immunodeficiency and a history of coccidioidomycosis may not develop a delayed-type hypersensitivity response to Spherusol ®.( 1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- Multi-dose vial (1 mL) containing a solution of spherule-derived C. immitis antigen, 1.27 mcg per 0.1mL. ( 3)
CONTRAINDICATIONS
- Severe allergic reaction (e.g., anaphylaxis) to Spherusol ®, or any component of Spherusol ® or other coccidioidin products. ( 4)
WARNINGS AND PRECAUTIONS
- Acute hypersensitivity reactions and anaphylaxis have occurred following the administration of other skin test antigens and may occur in individuals following the administration of Spherusol ®. ( 5.1)
- Patients receiving beta-blocking drugs may be refractive to the usual dose of epinephrine in cases of hypersensitivity. ( 5.2)
- Any condition or agent that impairs or attenuates delayed type hypersensitivity reactions, including infections and use of immunosuppressive drugs, can potentially cause a false negative reaction to Spherusol ®. ( 5.3)
ADVERSE REACTIONS
- The most commonly reported local adverse reactions were itching and swelling (>75%) and pain (>15%) within 7 days of administration.( 6.1)
- To report SUSPECTED ADVERSE REACTIONS, contact Nielsen Biosciences, Inc. at (855) 855-1212 or Food and Drug Administration (FDA) at 1-800-332-1088 or www.vaers.hhs.gov. ( 6.3)
DRUG INTERACTIONS
- Corticosteroids and immunosuppressive agents may suppress the response to the skin test.( 7.1)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2014
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING
- The expected response to Spherusol ® is a local area of inflammation at the site of the skin test. The reaction is usually dime to quarter size reaching maximum diameter between 24 and 48 hours. Larger accelerated reactions can occur, which may require treatment with local cold compresses and anti-inflammatory medication. ( 2.3, 6.1)
- Systemic reactions can occur with skin test antigens and in certain individuals these reactions may be life-threatening or cause death. Emergency measures and personnel trained in their use should be immediately available. Patients should be observed for at least 20 minutes following the administration of a skin test. ( 6.2)
- Spherusol ® should never be given intravenously. ( 5)
- To report SUSPECTED ADVERSE REACTIONS, contact Nielsen BioSciences, Inc. at (855) 855-1212 or MEDWATCH, Food and Drug Administration (FDA), 5600 Fishers Lane, Rockville, MD 20852-9782. Telephone: (800) 332-1088 or www.vaers.hhs.gov. ( 6.2)
-
1 INDICATIONS AND USAGE
Spherusol ® is a skin test antigen indicated for the detection of delayed-type hypersensitivity to Coccidioides immitis in individuals with a history of pulmonary coccidioidomycosis. Spherusol ® is approved for use in individuals 18-64 years of age.
- The use of Spherusol ® to detect delayed-type hypersensitivity responses in a general population with unknown exposure to C. immitis has not been evaluated.
- Persons with acute or disseminated coccidioidomycosis may not develop a delayed-type hypersensitivity response to Spherusol ®.
- Persons with immunodeficiency and a history of coccidioidomycosis may not develop a delayed-type hypersensitivity response to Spherusol ®.
-
2 DOSAGE AND ADMINISTRATION
2.1 Preparation for Administration
Spherusol ® is a clear, colorless sterile solution for intradermal administration.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If any of these conditions exists, the skin test antigen should not be administered.
2.2 Administration:
Spherusol ® is administered as a 0.1 mL dose by intradermal injection to the volar surface of the forearm using a tuberculin syringe (0.5 or 1.0 mL) and a ½ inch 26-27 gauge needle. The needle should be inserted bevel side up in the skin at a 15-20 degree angle. Intradermal injection of 0.1 mL Spherusol ® will result in a bleb 5-10 mm in diameter at the injection site.
2.3 Skin Test Assessment
The injection site should be assessed for induration at 48 hours (±4 hours) following administration. The response to the skin test should be measured by taking the mean of the orthogonal diameters of the area of induration. A mean induration of ≥ 5 mm is considered a positive delayed-type hypersensitivity response to Spherusol ®.
Repeat administration of Spherusol ® has not been evaluated.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Prevention and Management of Acute Hypersensitivity Reactions
Prior to administration, the healthcare provider should review the medical history for possible skin test sensitivity and previous skin test related adverse reactions to assess the risks and benefits. Immediate hypersensitivity, to include severe systemic reactions, may occur following administration of skin test antigens. Medications and equipment to manage possible anaphylactic reactions should be available for immediate use. Patients should be observed for a minimum of 30 minutes following administration to assess for adverse reactions.
5.2 Patients on Beta Blockers
Patients receiving beta blockers may be unresponsive to the usual doses of epinephrine used to treat serious systemic reactions, including anaphylaxis.
5.3 Immunosuppression
Any condition or agent that impairs or attenuates delayed-type hypersensitivity reactions, including infections and use of immunosuppressive drugs, can potentially cause a false negative reaction to Spherusol ®. [see Drug Interactions ( 7.1)]
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a skin test antigen cannot be directly compared to rates in the clinical trials of another skin test antigen, and may not reflect the rates observed in practice. As with any skin test antigen, there is the possibility that broad use of Spherusol ® could reveal adverse reactions not observed in clinical trials.
In a double-blinded placebo-controlled clinical trial conducted in areas of the U.S. endemic for C. immitis (Bakersfield, CA and Tucson, AZ), 54 adults (23-64 years of age) with a history of pulmonary coccidioidomycosis of at least 45 days duration, diagnosed by clinical findings, radiography and serological and/or mycological evidence of the disease, received a single dose of Spherusol ® concomitantly with two licensed skin test extracts (Candin ® and Trichophyton) and two controls (product diluent [thimerosal ≤0.0001%] and saline). Each intradermal injection of 0.1 mL of reagent was given at pre-determined sites on the right and left forearms. Solicited local adverse reactions and systemic adverse events occurring within 7 days after injection were recorded by study subjects via diary card. These events were also recorded on case report forms (CRFs) by study personnel during clinical visits 48 hours and 7 days following injections. Diary cards and CRFs did not record solicited local reactions by specific site. Local adverse reactions and systemic adverse events that occurred within 7 days were monitored until resolution. Reports of unsolicited adverse events and serious adverse events that occurred within 7 days after administration were collected on the diary cards or reported at study visits.
Table 1 lists the percentage of subjects reporting solicited local reactions (at any site) and solicited systemic adverse events within 7 days following the administration of Spherusol ®, Candin ®, Trichophyton, diluent control and placebo control.
Table 1. Frequency of Solicited Local Reactions and Systemic Adverse Events within 7 days of Administration of Spherusol ®, Candin ®, Trichophyton, Diluent Control and Saline Control in Subjects with a History of Pulmonary Coccidioidomycosis (N=53) Symptom Frequency (%) Local ∗ Any Mild Moderate Severe Itching 85 36 47 2 Swelling 79 36 41 2 Pain 17 13 4 0 Necrosis/Ulceration 4 2 0 2 Systemic Increased heart rate 4 2 2 0 Weakness 6 2 4 0 Faintness 0 0 0 0 Dizziness 2 2 0 0 Nausea/cramps 2 2 0 0 Flu-like symptoms 7 2 6 0 Difficulty breathing/shortness of breath 0 0 0 0 Any = Percentage of subjects experiencing adverse event of any intensity; Mild = Barely noticeable, not bothersome; Moderate = Distinctly noticeable discomfort; Severe = Needs medical attention. ∗ Local reactions occurring at any injection site Of subjects with severe reactions, one subject required treatment with oral corticosteroids for ulceration and swelling. Based on investigator's determination the reaction was at the site of Trichophyton injection. All severe reactions resolved without sequelae.
During the 7 days following administration two subjects reported unsolicited adverse events: one subject reported joint pain, fatigue, cough, sensitivity at a test site (test site not specified), and one subject with erythema immediately after administration (test site not specified). The intensities of these unsolicited adverse events were not recorded.
No serious adverse events or deaths were reported during the clinical study.
-
7 DRUG INTERACTIONS
7.1 Corticosteroids and Immunosuppressives
Corticosteroids and Immunosuppressive agents may suppress the response to the skin test. Pharmacologic doses of corticosteroids may suppress the response to skin test antigens after two weeks of therapy. The mechanism of suppression is thought to involve a decrease in monocytes and lymphocytes, particularly T-cells. The normal DTH response usually returns to pre-treatment levels within several weeks after steroid therapy is discontinued. (5) The use of Spherusol ® has not been evaluated during or following the use of corticosteroids or immunosuppressive agents.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
The safety and effectiveness of Spherusol ® in pregnant women have not been established.
Pregnancy Category C
Animal reproduction studies have not been conducted with Spherusol ®. It is also not known whether Spherusol ® can cause fetal harm when administered to a pregnant woman or affect reproduction capacity. Spherusol ® should be given to a pregnant woman only if clearly needed.
8.2 Labor and Delivery
No information is available to assess the effects of Spherusol ® on childbirth.
8.3 Nursing Mothers
The safety and effectiveness of Spherusol ® in nursing women have not been established.
It is not known whether Spherusol ® is excreted in human milk. However, because the potential exists for Spherusol ® to be excreted in human milk, caution should be exercised when administering Spherusol ® to a nursing woman.
-
11 DESCRIPTION
Spherusol ® is a sterile aqueous solution of extracts of C. immitis spherules. The multi-dose vial contains 0.9% sodium chloride and 0.014% sodium borate with 0.4% phenol as a preservative. Residual thimerosal from the manufacturing process is present at a concentration of ≤0.0001% (<0.05 mcg mercury/0.1 mL dose). Each 0.1 mL dose contains 1.27 mcg of spherule-derived antigen.
The potency of each lot of Spherusol ® is determined in sensitized guinea pigs.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
In individuals with a history of pulmonary coccidioidomycosis Spherusol ® is thought to elicit a cellular immune reaction to C. immitis, as evidenced by a delayed-type hypersensitivity (DTH) response to Coccidioidin (1,2,3,4). The general mechanism of the DTH response is based on the interaction of antigen with CD 4 and CD 8 lymphocytes followed by the secretion of interleukins and other lymphokines from macrophage cells. The release of effector molecules causes endothelial cells lining the blood vessels to become permeable and allows fibrinogen to escape into the surrounding tissue where it is converted to fibrin. The deposition of fibrin and the accumulation of T-cells and monocytes within the extracellular spaces cause the tissue to swell and become indurated. This process is usually detectable in 18 hours and peaks at 48 hours. (5)
-
14 CLINICAL STUDIES
The delayed-type hypersensitivity response following administration of Spherusol ® was evaluated in one U.S. study which enrolled persons with a history of coccidioidomycosis. Two further U.S. studies enrolled subjects without a history of coccidioidomycosis; in one of these studies subjects had a history of histoplasmosis. In each study, concomitant with Spherusol ®, two additional skin test extracts, Candin ® and Trichophyton (positive controls), were administered along with a saline (negative control) and a diluent containing ≤ 0.0001% thimerosal (negative control). All skin tests and controls were administered as 0.1 mL doses in a randomized pattern on the volar surface of the forearms. Investigators and subjects were blinded to identity and placement of skin test antigens and controls. Responses were read at 48 hours (±4 hours) following administration. Induration responses were measured for each test site and recorded as the mean of the orthogonal diameters. A positive skin test was defined as a mean induration of ≥ 5 mm at 48 hours following administration of the antigens or controls. For each subject Spherusol ® skin test results were considered valid if ≥ 5 mm was observed at the positive control antigen sites and no induration ≥ 5 mm was observed at the negative control sites.
The use of Spherusol ® to detect delayed-type hypersensitivity response in a general population with unknown exposure to C. immitis has not been evaluated.
14.1 Induration Response in Subjects With a History of Pulmonary Coccidioidomycosis
A multicenter, double-blinded study in endemic areas (Bakersfield, CA and Tucson, AZ) enrolled 54 adults with a history of pulmonary coccidioidomycosis diagnosed by radiography, laboratory serologies (e.g., complement fixation, immunodiffusion) and/or culture. Subjects were 23-64 years of age; 28% women; 70% Caucasian, 11% Hispanic, 2% Asian, 2% Native American and 4% who did not specify race or ethnicity. Of the 51 subjects with valid skin test results, 50 subjects [98.0%; 2-sided 95% CI (89.6%, 100%)] had a mean induration of ≥ 5 mm at the Spherusol ® injection site. Among subjects with valid skin test results the average size of induration at the Spherusol ® injection site was 17 mm (range 5 mm-39 mm).
The receipt of concurrent or previous antifungal therapy did not appear to interfere with or accentuate the induration response to Spherusol ®.
14.2 Induration Response in Subjects Without a History of Pulmonary Coccidioidomycosis or Known Exposure to C. immitis.
A single site, double-blind study conducted in a non-endemic area for C. immitis (Spokane, WA) enrolled 60 adult subjects (18-56 years of age) with no known exposure to C. immitis by travel to or residency in an endemic area. Subjects had negative serologies to C. immitis by complement fixation, immunodiffusion and/or ELISA. Subjects enrolled in the study were 65% women; 96% Caucasian, 2% Hispanic and 2% Native American. At the 48 hour (± 4 hours) assessment, a total of 55 subjects had valid skin test results (5 subjects had negative skin test results to all reagents and were considered un-interpretable). One subject (1/55) had a 5 mm mean induration response to Spherusol ® and 54 subjects with demonstrated negative responses (< 5mm mean induration) to Spherusol ®. Fifty-four of the 55 subjects with valid skin test responses [98.2%; 2-sided 95% CI (90.3%, > 99.9%)] demonstrated a negative induration response to Spherusol ®. When the five subjects who had un-interpretable responses were analyzed as if these responses represented positive reactions to Spherusol ®, 54 of 60 subjects 90.0% (CI 79.5%, 96.2%) demonstrated a negative induration response.
14.3 Induration Response in Subjects With a History of Pulmonary Histoplasmosis
A single site, double-blind study conducted in a non-endemic area for C. immitis, but endemic for H. capsulatum (Blair, NE) enrolled 12 adult subjects (33 to 60 years of age) with no known exposure to C. immitis by travel to or residency in an endemic area. All subjects had a history of pulmonary Histoplasmosis. Subjects had negative serologies to C. immitis by complement fixation, immunodiffusion and/or ELISA. Subjects were 42% women and 100% Caucasian. At the 48 hour (± 4 hours) assessment, all 12 subjects reacted to at least one of the positive controls with ≥ 5mm mean induration and demonstrated negative (< 5mm) induration responses to thimerosal and saline controls. No positive induration responses to Spherusol ® were observed [1-sided 97.5% CI; (0%, 26.5%)] among subjects who had a previous history of disease caused by H. capsulatum and no history of travel to areas endemic for C. immitis. These findings support the lack of cross-reaction between the cellular immune responses induced by the two fungal species.
-
15 REFERENCES
- Edwards, PQ and Palmer CE. Prevalence of sensitivity to coccodioidin, with special reference to specific and nonspecific reactions to coccidioidin. Dis Chest. 31:35. 35-60, 1957.
- Emmons, C.W., Binford, C. H., Ulz, J.P. Kwon-Chung, K.J. Medical Mycology, Leanne Febiger, Philadelphia, Chapter 17, 230, 1977.
- Emmons CW and Olson BJ. Studies of the role of fungi in pulmonary disease. I. Cross-reactions with histoplasmin. Pub Health Rep. 60(47):1383, 1945.
- Levine HB, Restrepon A, Eyck DR, and Stevens DA. Spherulin and coccidioidin: cross-reactions in dermal sensitivity to histoplasmin and paracoccidioidin. Am J Epidemiol. 101(6):512, 1975.
- Zweiman, B. Cell-mediated immunity in health and disease. Allergy Principles and Practices, Mosby, Saint Louis, Chapter 50: 696, 1998.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
-
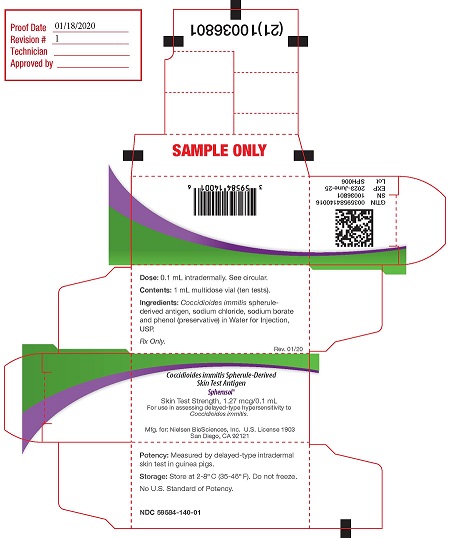
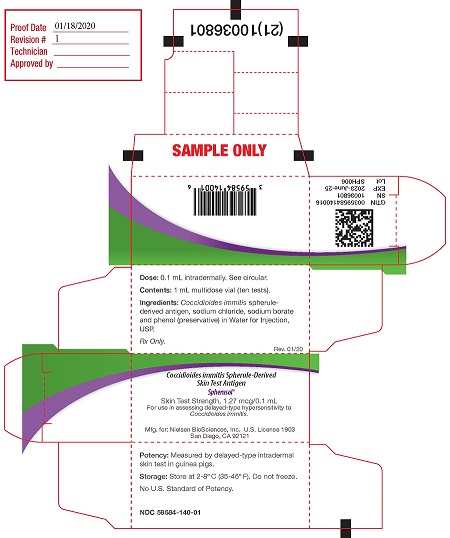
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 1 mL Vial
NDC 59584-140-01
Coccidioides immitis Spherule-Derived Skin Test Antigen
Spherusol ®
Vol:1mL (10 Tests)
Dose:0.1mL Intradermally
See Package Insert
Store: 2-8°C Rx only
Preservative: 0.4% Phenol
Mfd for Nielsen BioSciences, Inc.
San Diego, CA. 92121
Lot: 000000 (to be inserted vertically)
Exp: (to be inserted vertically)

-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - 1 mL Carton
Dose: 0.1 mL intradermally. See circular. (1st panel)
Contents: 1 mL multidose vial (10 tests)
Ingredients: Coccidioides immitis spherule-derived antigen, sodium chloride, sodium borate and phenol (preservative) in Water for Injection, USP.
Rx only.
Rev. 07/19Coccidioides immitis Spherule-Derived Skin Test Antigen (panel 2)
Spherusol ®
Skin Test Strength 1.27 mcg/0.1 mL
For use in assessing delayed-type hypersensitivity to Coccidioides immitis
Nielsen BioSciences, Inc, U.S. License 1903
Mfd. by:Allermed Laboratories, Inc. U.S. License 0467
San Diego CA 92121Potency: Measured by delayed-type intradermal skin tests in guinea pigs. (panel 3)
Storage: Store at 2 - 8°C (36 - 46°F). Do not freeze.
No U.S. Standard of Potency.
2D GS1 serialized barcode (on end flap)
GTIN (end flap)
SN (end flap)
EXP (end flap)
Lot (end flap)
-
INGREDIENTS AND APPEARANCE
SPHERUSOL
coccidioides immitis spherule-derived skin test antigen injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59584-140 Route of Administration INTRADERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COCCIDIOIDES IMMITIS SPHERULE (UNII: ITY7G7Q744) (COCCIDIOIDES IMMITIS SPHERULE - UNII:ITY7G7Q744) COCCIDIOIDES IMMITIS SPHERULE 12.7 ug in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) .009 g in 1 mL SODIUM BORATE (UNII: 91MBZ8H3QO) .00014 g in 1 mL PHENOL (UNII: 339NCG44TV) .0045 mL in 1 mL WATER (UNII: 059QF0KO0R) .99 mL in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59584-140-01 1 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product 06/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125354 07/29/2011 Labeler - Nielsen BioSciences, Inc. (078835288) Registrant - Nielsen BioSciences, Inc. (078835288) Establishment Name Address ID/FEI Business Operations Allermed Laboratories, Inc. 073364531 manufacture(59584-140)