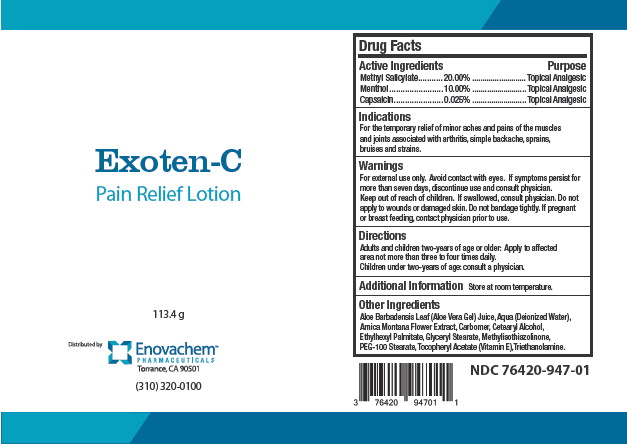
Label: EXOTEN-C- methyl salicylate, menthol, capsaicin lotion
- NDC Code(s): 76420-947-01
- Packager: Asclemed USA, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Indications
- Warnings
- Directions
- Additional Information
- Other Ingredients
- Principal Display Panel - Exoten-C Label
-
INGREDIENTS AND APPEARANCE
EXOTEN-C
methyl salicylate, menthol, capsaicin lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76420-947 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 20 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 10 g in 100 g CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.025 g in 100 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ETHYLHEXYL PALMITATE (UNII: 2865993309) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) PEG-100 STEARATE (UNII: YD01N1999R) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76420-947-01 113.4 g in 1 BOTTLE; Type 0: Not a Combination Product 02/10/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 02/10/2017 Labeler - Asclemed USA, Inc. (059888437)